経営戦略
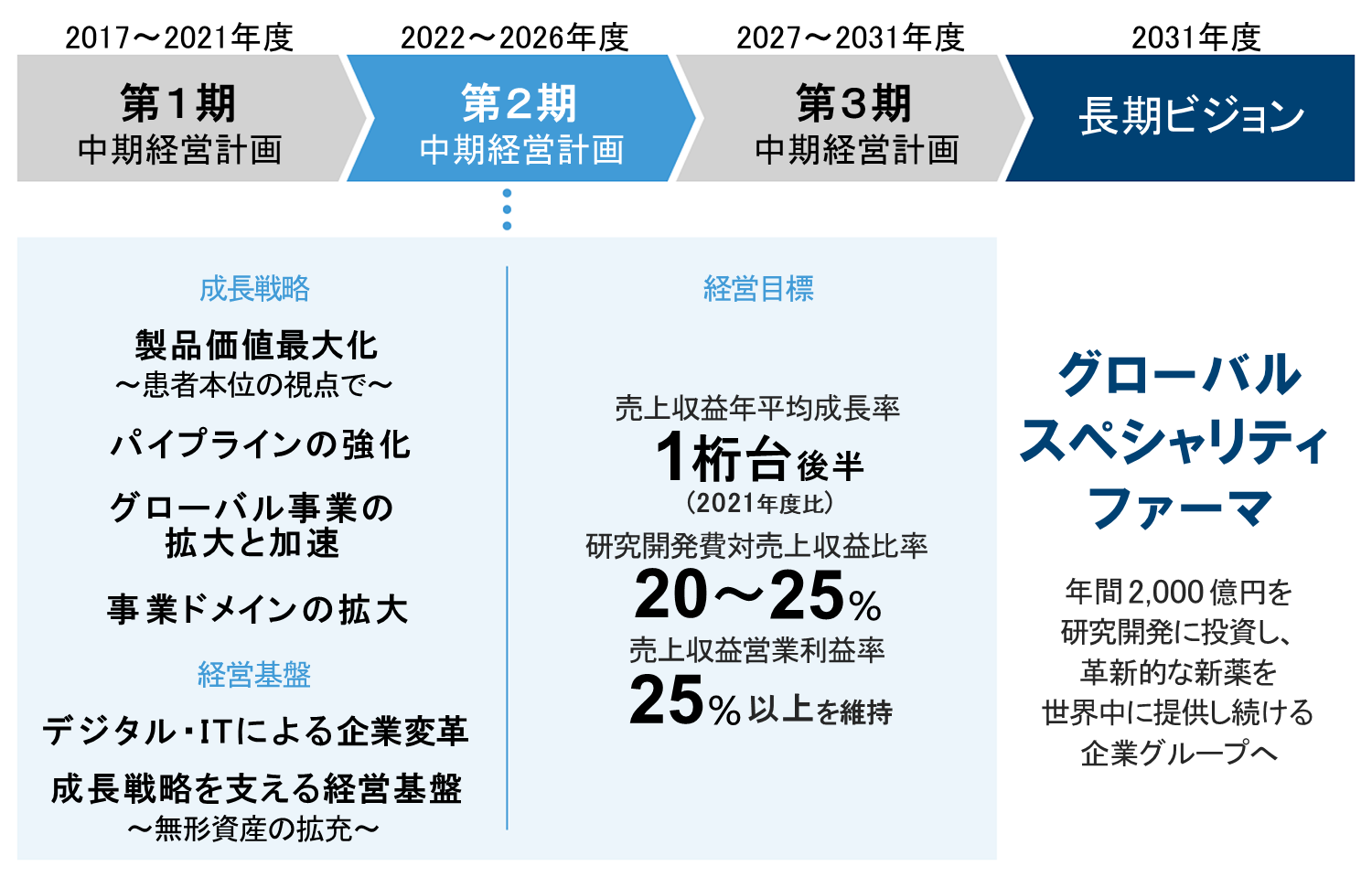
当社は2031年度をゴールとする長期ビジョンを策定しています。その実現に向けて中期的な成長戦略を定めるとともに、財務・非財務両面を包括したマテリアリティを特定し、持続的な成長を図っています。長期ビジョンにおいて目指すのは、国内製薬大手と同程度の年間2,000億円を研究開発に投資し、革新的な新薬を世界中に提供し続ける「グローバルスペシャリティファーマ」です。その実現に向けて15年間を3つにわけ、各5年の中期経営計画を進めています。2022年度からは第2期の中期経営計画を開始。3つの経営目標の達成に向けて、柱となる4つの成長戦略を推進するとともに、DXや人財などの経営基盤強化にも取り組んでいます。
サステナブル経営方針
創業から300余年、私たちは社会とともに歩んできました。
「病気で苦しむ人を救いたい」という想いを実現するため、不可能と思われていた革新的な新薬を次々と創出してきました。私たちはこれからも、企業理念の実践を通じて人々の健康に貢献するとともに、責任ある事業活動を通して、持続可能な社会の実現に挑戦し続けます。
人々の健康への貢献
- 自社創薬に加えて、世界のトップサイエンティストと協働して医薬品の研究開発に挑戦し、独創的かつ革新的な医薬品を安心・安全・適切に患者さんに提供することによって、世界の患者さんやその家族に多くの希望を届けます。
- エビデンスに基づいた次世代ヘルスケア事業によって、人々がより健やかに生活できる社会の実現に貢献します。
成長戦略(第2期 中期経営計画)
経営基盤を強化し、4つの成長戦略を実践
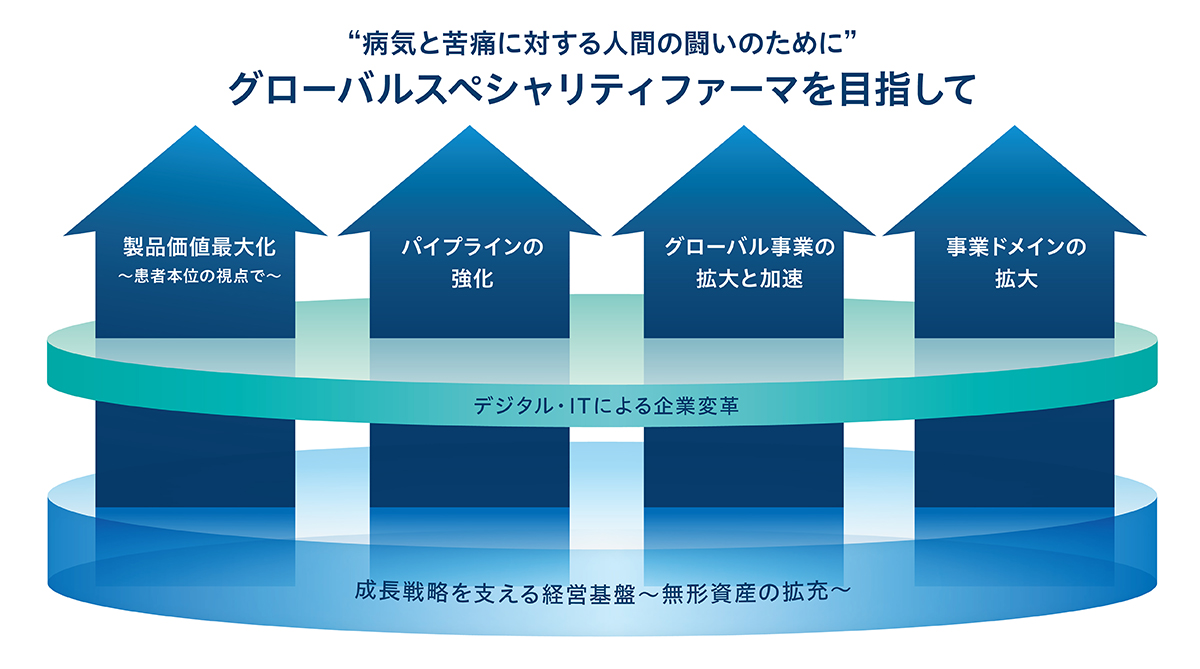
「製品価値最大化~患者本位の視点で~」
患者さんとそのご家族のウェルビーイング(心身的・社会的・生活満足度が満たされている状態)の実現に、医療従事者と共に挑み、その結果として新薬が医療現場に速やかに浸透している状態を目指しています。
マーケティングでは医療課題に対して医療従事者とともに患者さん視点で取り組む⼈財を育成するとともに、デジタルを活用して効果的かつ効率的な情報提供・収集を行うことで、製品価値を最大限引き出せるように取り組んでいます。
開発では重要戦略分野であるオンコロジー領域を中心に100近くの臨床試験を実施しており、引き続き製品価値の最大化を目指しています。
「パイプラインの強化」
当社は「グローバルスペシャリティファーマ」を目指し、医療ニーズの高い、がんや免疫疾患、神経疾患、スペシャリティ領域において、疾患ノウハウを蓄積し、革新的な医薬品の創出に取り組んでいます。また、世界をリードする大学や研究機関、バイオベンチャー企業との研究・創薬提携を強化し、ファーストインクラスが狙える独自性の高いパイプラインの充実を図っています。さらに、創薬シーズに応じて最適な創薬モダリティを選択し、独自性の高い自社創薬を進めるとともに、非臨床や臨床試験のデータを用いた創薬標的の検証やトランスレーショナル研究を強化し、研究開発の確実性の向上に努めています。加えて、医療ニーズの高い分野での革新的な化合物の導入や新技術の獲得も積極的に進めることでパイプラインの強化を図っています。
「グローバル事業の拡大と加速」
新薬を世界中に提供できるよう、グローバル事業の拡大に取り組んでいます。海外事業を拡大・加速させるために、デサイフェラ社を買収し、パイプラインと研究開発力を強化するとともに、米欧での販売基盤を獲得しました。さらに、デサイフェラ社を米欧事業における拠点として、従来、米国および英国に有していた機能を再編し、集約します。これにより、当社グループ一体となってグローバル事業を一層加速させていきます。短期的には、QINLOCKとROMVIMZAの適応追加や販売地域拡大を通じて製品価値を最大化し、ONO-4059(ベレキシブル錠)の米国での上市に向けた活動を推進します。また、グローバルでの開発体制の強化に取り組み、既存のがん領域に加えて他の疾患領域においても、米欧での開発を推進します。今後も革新的医薬品を世界中のより多くの患者さんに速やかに届けられるよう取り組んでまいります。
「事業ドメインの拡大」
拡大するヘルスケア分野のニーズを捉え、新たな価値を提供し続けるために事業ドメインの拡大に取り組んでいます。機能性表示食品睡眠サプリメント「REMWELL(レムウェル)」については、継続的に顧客の拡大を進めています。また、がん(大腸がん、胃がん、肺がん、乳がん)患者さんの告知直後の心のケアや医師の話を理解するためのヘルスケアリテラシー向上をサポートするツール「michiteku」β版に加えて、2025年1月には、日常と治療の両立を支援する通院日管理アプリ「michiteku YOHA」の提供を開始しました。さらにこれらの活動と並行して、小野デジタルヘルス投資合同会社による、ヘルスケア分野でのベンチャー企業への投資活動を通じて新たな事業の創出と拡大を目指しています。
「デジタル・ITによる企業変革」
デジタル・ITの活用を機能横断的に推し進め、成長戦略の加速、事業プロセスの革新、新たな価値創造(DX)を実現できる企業への変革を目指します。そのために、社内外のデータ活用環境と独自の視点によるデータ分析能力、最新テクノロ ジーに支えられた柔軟なIT基盤の整備を進めています。社内外のデータを活用し、ビジネス上の課題や新しい機会を適時的確に検知・判断し、ビジネス変革の構想に反映・実装していきます。
「成長戦略を支える経営基盤〜無形資産の拡充〜」
人的資本、企業ブランド、デジタル・IT基盤等の無形資産の拡充により、4つの成長戦略を支え、飛躍的な成長を目指します。人的資本の拡充では、事業の成長を推進するための人財の確保・育成を進めるとともに、高い従業員エンゲージメントを実現するための組織風土・カルチャーの醸成を推進しています。また、企業ブランドについては、グローバルでの企業ブランドの浸透をデサイフェラ社とともに進めることで、企業価値の向上に取り組みます。
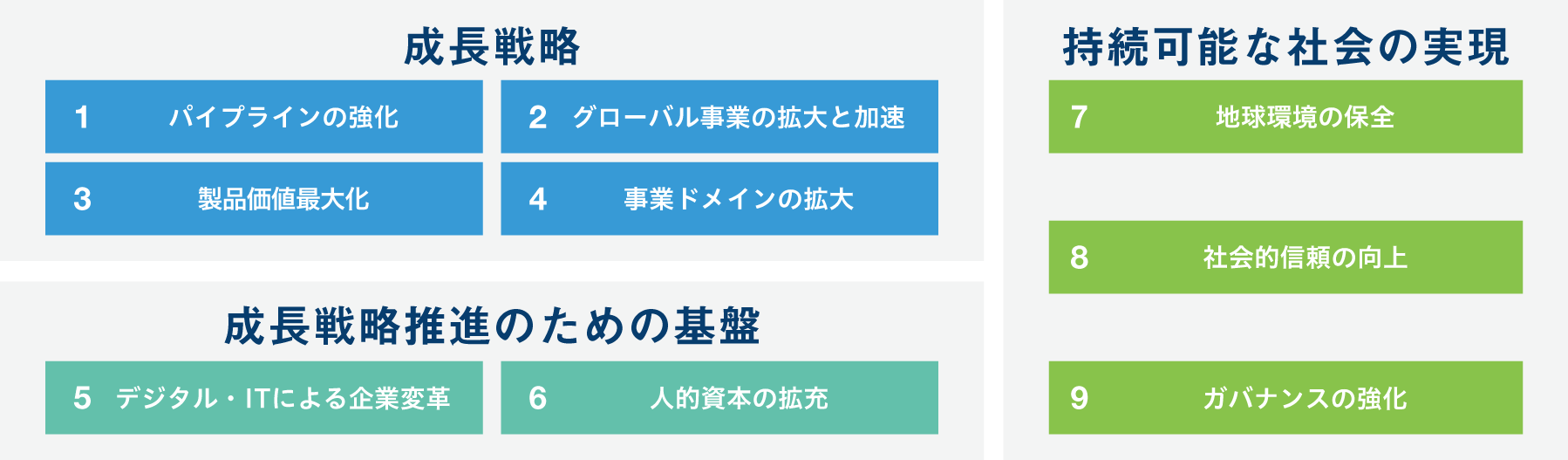
マテリアリティ(経営の重要課題)
財務と非財務を統合した「経営の重要課題」に取り組む
サステナブル経営方針と成長戦略のもと、財務と非財務を統合した経営を推進していくためにマテリアリティを特定しています。マテリアリティごとに取り組みを推進することで、当社と社会、双方の持続可能性向上を目指します。
さらに2024年6月に米国Deciphera Pharmaceuticals社が小野薬品グループに加わり、欧米での自販が可能になったことを契機として、マテリアリティの見直しを実施しました。外部ステークホルダーの声も取り入れ、2025年3月に18項目のマテリアリティを9項目に集約しました。
|
|
中長期の目指す姿 |
|---|---|
|
1 |
・トップサイエンティストと協働して世界を変える新薬づくりを加速し、新薬候補のPOC確立のスピードと精度を向上させるとともに、ライセンス活動によりパイプラインが拡充している。 |
|
2 |
・世界で闘えるスペシャリティファーマとして、グローバルでの事業拡大を加速している。 |
|
3 |
・患者さんとそのご家族のウェルビーイング実現に医療従事者とともに挑み、その結果として新薬が速やかに浸透している。 |
|
4 |
・デジタルや当社の強みを活用し、社会課題の解決、次世代ヘルスケアの実現に貢献する。 |
|
5 |
・セキュアなグローバルIT基盤を整備するとともに、デジタルによる企業変革を実現している。 |
|
6 |
・企業理念・ビジョンの実現に向けた人財戦略に基づき、事業の成長に資する人財の採用と育成、そして多様性の向上と一体感の醸成につながる組織風土の実現に向けて取り組みを進めている。人財を惹きつける制度・施策が定着しており、かつ全ての社員が安心・安全に働くことのできる環境が提供されている。 |
|
7 |
・人々が健康で健全な社会を迎えらえるよう、「ECO VISION 2050」のもと、製薬業界における環境リーディングカンパニーを目指し、次世代へ豊かな地球環境を引継ぐことに努める。 |
|
8 |
・品質保証および安全管理の業務を適正に行うとともに、患者さんに当社製品を安定的かつ継続的に改善しながら供給する。 ・国連の「ビジネスと人権に関する指導原則」に基づいたマネジメントを実践するとともに、ビジネスパートナーのサステナビリティ関連リスクを把握し、持続可能な社会の実現を目指して共に取り組んでいる。 ・希少疾患や小児疾患に対する革新的医薬品の提供と医療インフラの未成熟な地域での医療基盤整備に貢献する。 |
|
9 |
・コンプライアンス違反の未然防止を実現するコンプライアンスリスク管理体制の確立など、持続的な成長を実現するための実効性あるガバナンス体制を構築する。 |
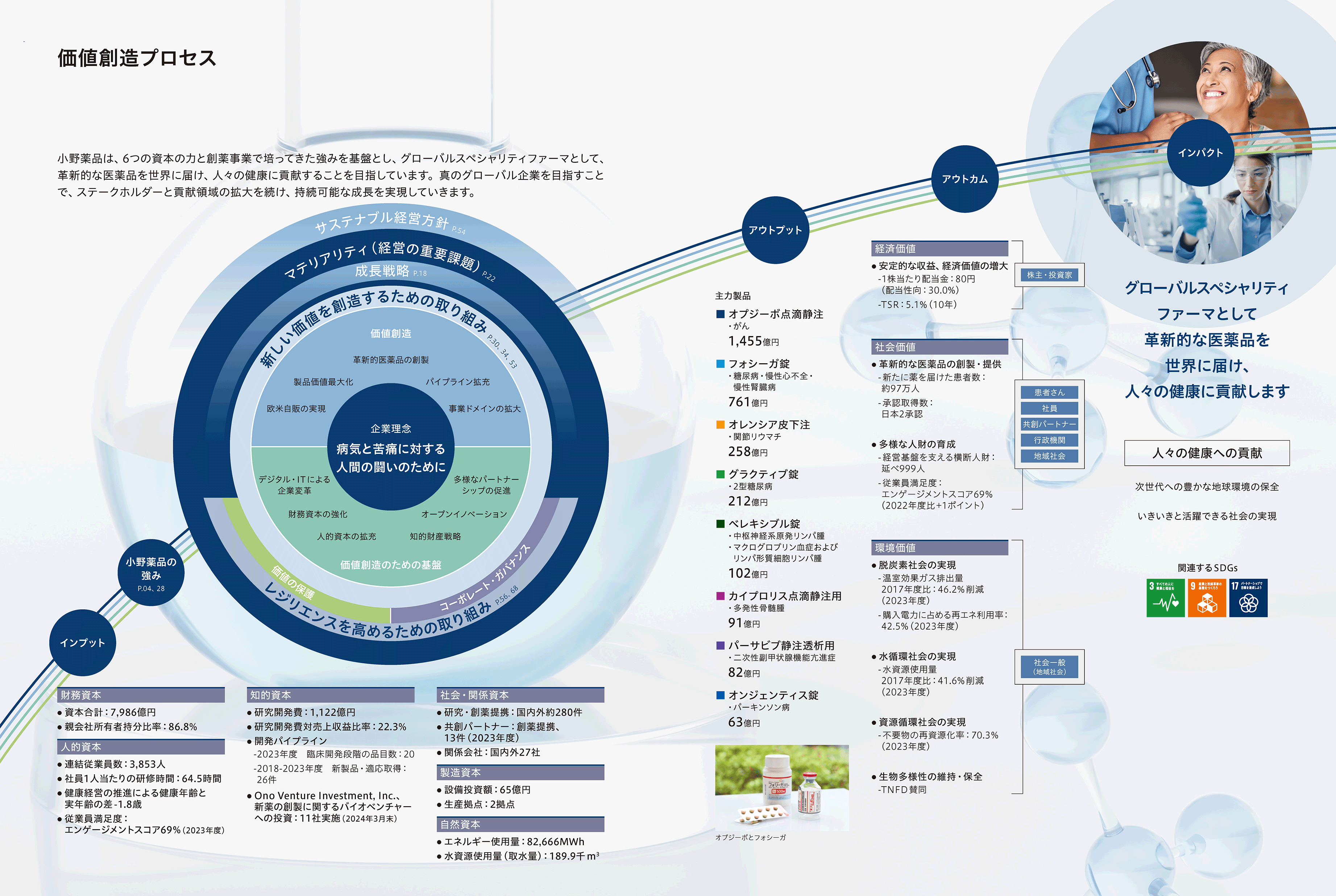
価値創造プロセス
独創的かつ革新的な医薬品を世界へ届けることで持続可能な社会を実現させます。
中長期投資方針
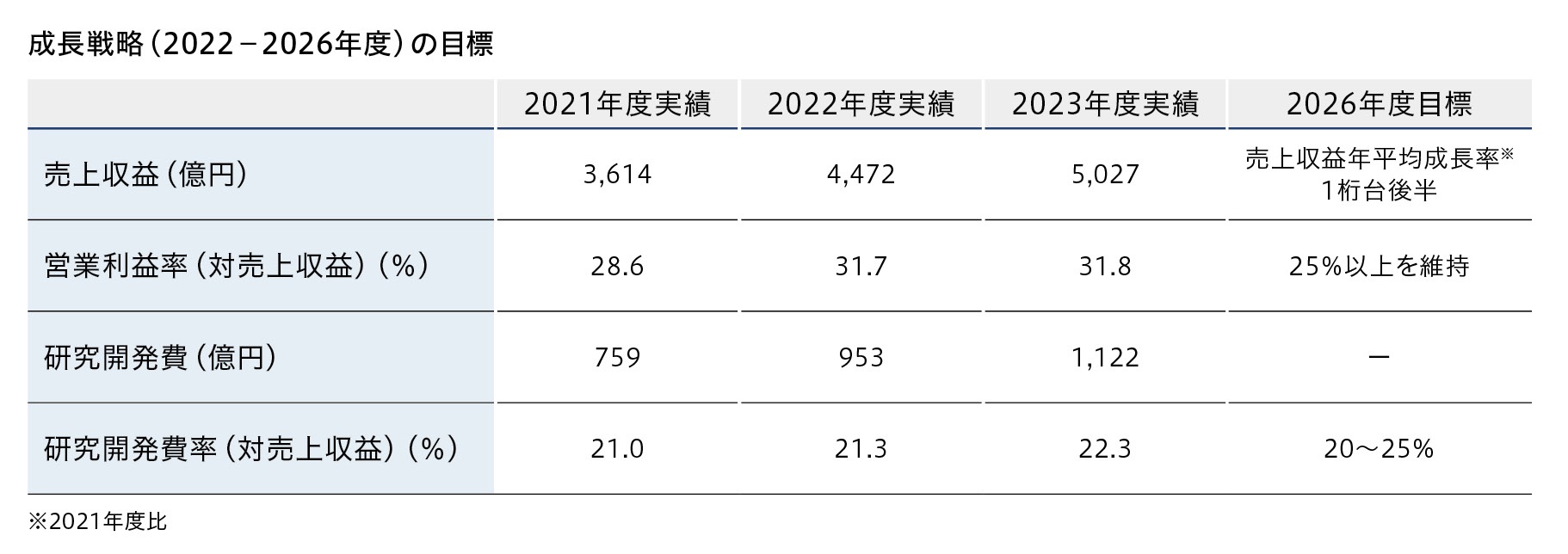
持続的な成長を目指して、研究開発などの戦略投資と株主還元をバランスよく行うことが中長期の財務方針です。売上収益拡大による営業キャッシュ・フローの継続的な充実による安定した投資原資を確保しつつ、政策保有株式縮減を通じた資産効率の向上にも取り組み、創出されたキャッシュは投資対効果を検討しながら研究開発をはじめとした成長のための投資に投下していきます。
2022年度から2026年度の5年間は、2021年度と比較して1桁台後半の年平均成長率で売上収益の拡大を図ります。
そして、売上収益の20~25%程度を研究開発に投資しつつ、営業利益率は25%以上を維持することを目指します。売上収益の拡大と積極的な研究開発投資によって利益拡大を図ることで、短期志向に陥ることなく株主資本コストを上回るROEを達成できると考えています。
成長の実現と財務基盤の健全性を確保しつつ、安定した株主還元も実施するために、2024年度より累進的な配当方針と配当性向40%をめど配当を行うことを目標としています。
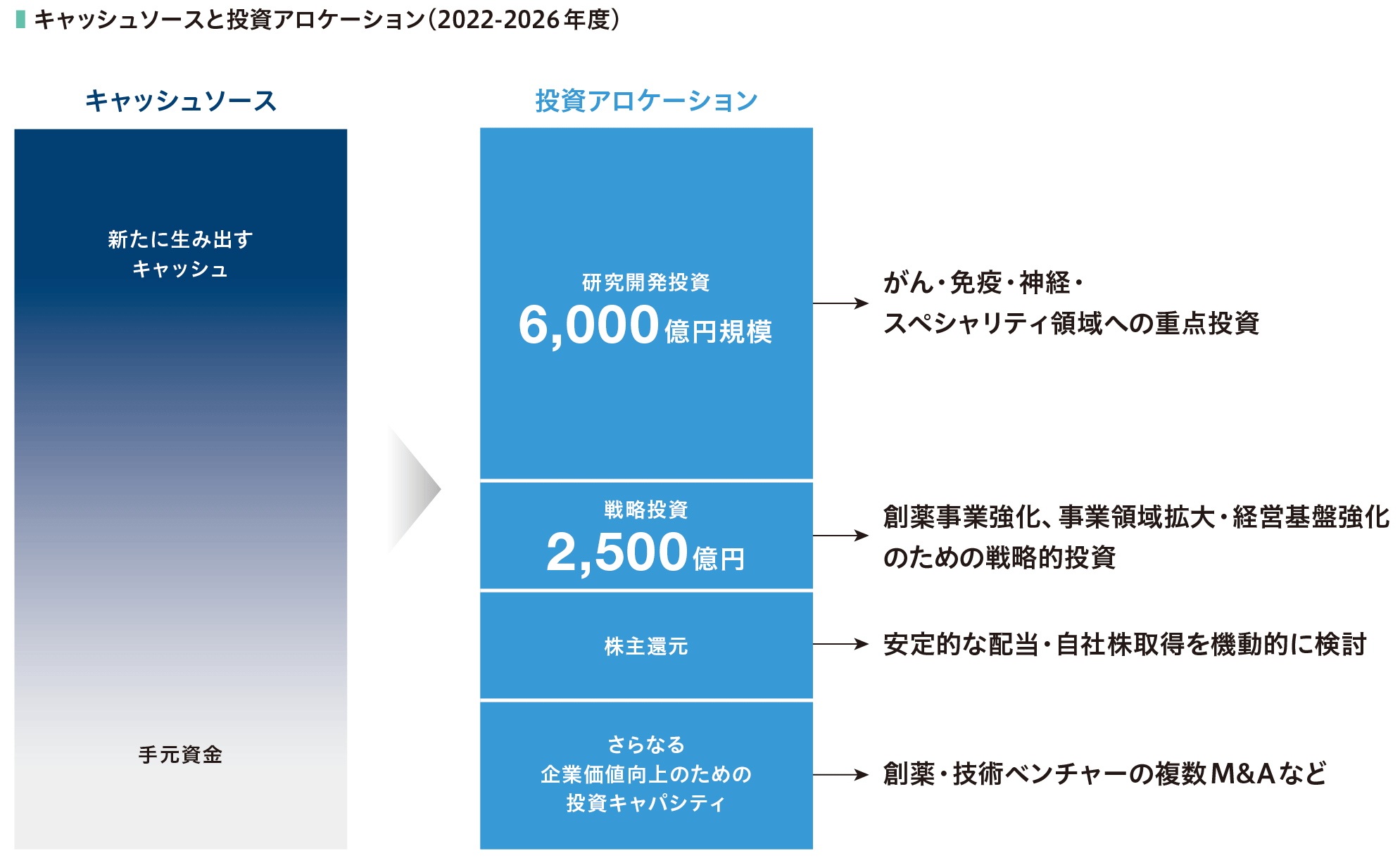
キャッシュアロケーション
2022年度からスタートした中期経営計画第2期のキャッシュアロケーションは、おおむねシナリオ通りに進捗しています。これまでの利益の積み上げと新たに生み出すキャッシュから6,000億円を研究開発投資に割くとともに、まだ十分に残っている投資余力を戦略投資枠として、創薬事業強化、事業領域拡大、経営基盤強化に対し、2,500億円を投資する方針で活動してきました。
短中期的には製品価値最大化のための活動、研究開発、そして株主還元をバランスよく行いつつ、余剰資金の可視化を進めています。これにより、パイプライン強化のための投資機会に迅速に対応できるようにしています。これら資金管理に加え、中長期的には政策保有株の流動化を進めることにより、手元資金を創出していきます。こうしたキャッシュアロケーションによる投資計画を引き続き着実に遂行することで、中期経営計画における成長戦略の実現が達成できると見込んでいます。特に、「欧米自販の実現」については、自社創薬のONO-4059やM&Aで現実のものとし、さらに複数の製品を欧米で自販するためのアセットも着実に整いつつあります。
2023年度は、研究開発費が初めて1,000億円を超え1,122億円となりました。2017年度に中期経営計画がスタートした際は575億円で約2倍となっており、高い研究開発力を生かして新薬パイプラインを拡充する活動は順調に進んでいます。成長投資についても、創薬事業強化ばかりでなく、事業領域拡大や経営基盤強化へ投資を行っているほか、2024年6月には米国製薬ベンチャーのDeciphera社を総額約24億米ドル(約3,800億円)で買収し、パイプラインの拡大のほか、米国と欧州における自販体制強化も着実に進めています。
資本コストと資本収益性
資本コストや株価を意識した経営の実現に向け、以下の通り取り組んでいます。
資本コストの把握
当社では資本コストとして、「株主資本コスト」を把握しています。
分析・評価
現在、当社は主力製品の特許切れという業界特有の課題を乗り越え、持続的な成長を実現するために、積極的な戦略投資を展開しています。現在は将来の成長に向けた投資の段階であり、短期的に資本収益性、ROEが低下しておりますが、これが市場の評価指標であるPBRが1倍前後で推移している理由であると分析しています。一方、当社の成長戦略は着実に進捗しています。
当社の成長戦略は2031年のオプジーボの特許切れを乗り越え、「グローバルスペシャリティファーマ」となることを目標としています。既存製品等の製品価値最大化を実現し、成長投資の原資を確保することに取り組み、売上も着実に計上しております。グループ一丸となってパイプラインの強化も行っております。そして、自ら欧米での臨床試験を成功させ、承認を得て、市場規模の大きい欧米での自販を実現し、グローバル展開することを目標としています。
2024年6月にデサイフェラ社を買収し、現在欧米をはじめ40数か国で承認されている消化管間質腫瘍治療剤キンロックと、2025年2月に米国で上市した腱滑膜巨細胞腫の新規治療剤ロンビムザの2製品を獲得しました。2025年7月には欧米の開発・販売拠点をデサイフェラ社に統合し、今後は同社を中心としてグローバル展開を推進・加速して参ります。
米国での上市に向けて中枢神経系原発リンパ腫を対象に開発を進めているベレキシブルは上市が見込める段階まで進捗しており、グローバル展開への寄与が期待されます。さらには真性多血症という希少疾患の治療薬候補であるサパブルセンも、その作用メカニズムから高い成功確率が見込まれる有望な化合物であり、グローバル展開の加速が大きく期待できます。現在実施中の約10本のPoC試験(Proof of Concept試験/開発の早期段階に行う臨床試験で、創薬段階で想定した安全性および有効性が臨床で発揮されるかを確認する試験)で評価中のパイプライン、あるいは継続的に進めているライセンス活動や買収を通じて獲得したパイプラインの中から、複数製品をグローバルで上市することができれば、オプジーボの特許切れを克服し、さらなる成長を確実なものとすることができます。
その上で、中長期的なインプライド株主資本コストの抑制のため、成長投資の成果実現を目指すだけでなく、ESGへの取り組みや経営リスクの軽減に寄与するガバナンス向上、人的資本への投資、従業員エンゲージメント向上への取り組みを引き続き進めます。
当社は、長期ビジョンで掲げる「グローバルスペシャリティファーマ」に向けた歩みを、着実に進めます。
継続的な取組み
今後も資本コストや市場の評価を意識した経営を推進し、積極的な開示および投資家との対話に努めていきます。なお、具体的な取り組みなどについては、当社コーポレートレポートにて開示しています。
コーポレートレポート(財務戦略と資源配分)
29~32ページを参照
各種関連指標(実績)
*税引後%
|
指標 |
2020年度 |
2021年度 |
2022年度 |
2023年度 |
2024年度 |
|
|---|---|---|---|---|---|---|
|
資本コスト |
株主資本コスト* |
6%程度を想定(CAPMベース) |
||||
|
資本収益性 |
ROE |
12.6% |
12.5% |
16.1% |
16.7% |
6.4% |
|
市場評価 |
PBR |
2.27倍 |
2.28倍 |
1.82倍 |
1.45倍 |
0.96倍倍 |
