グローバル展開

グローバルにおけるヘルスケアの未来を切り拓く挑戦
執行役員/事業戦略本部長
谷川 雅之
グローバルにおけるヘルスケアの未来を切り拓く挑戦

執行役員/事業戦略本部長
谷川 雅之
当社は、グローバルにおけるヘルスケア領域の新たな未来を切り拓くことに挑戦しています。すでに韓国と台湾で販売拠点を設立しており、次に欧米市場での自社販売体制を強化する第2段階に進んでいます。2024年には、Deciphera社を買収し、がん領域での新薬開発と販売力を飛躍的に向上させました。これにより、私たちの卓越した研究開発能力と販売力を駆使し、真のグローバルスペシャリティファーマとして、世界中の患者さんに革新的な治療の選択肢を提供します。新たな希望と未来をお届けすることを通じて、持続可能な成長を目指しています。
グローバル企業への成長に向けたステップ
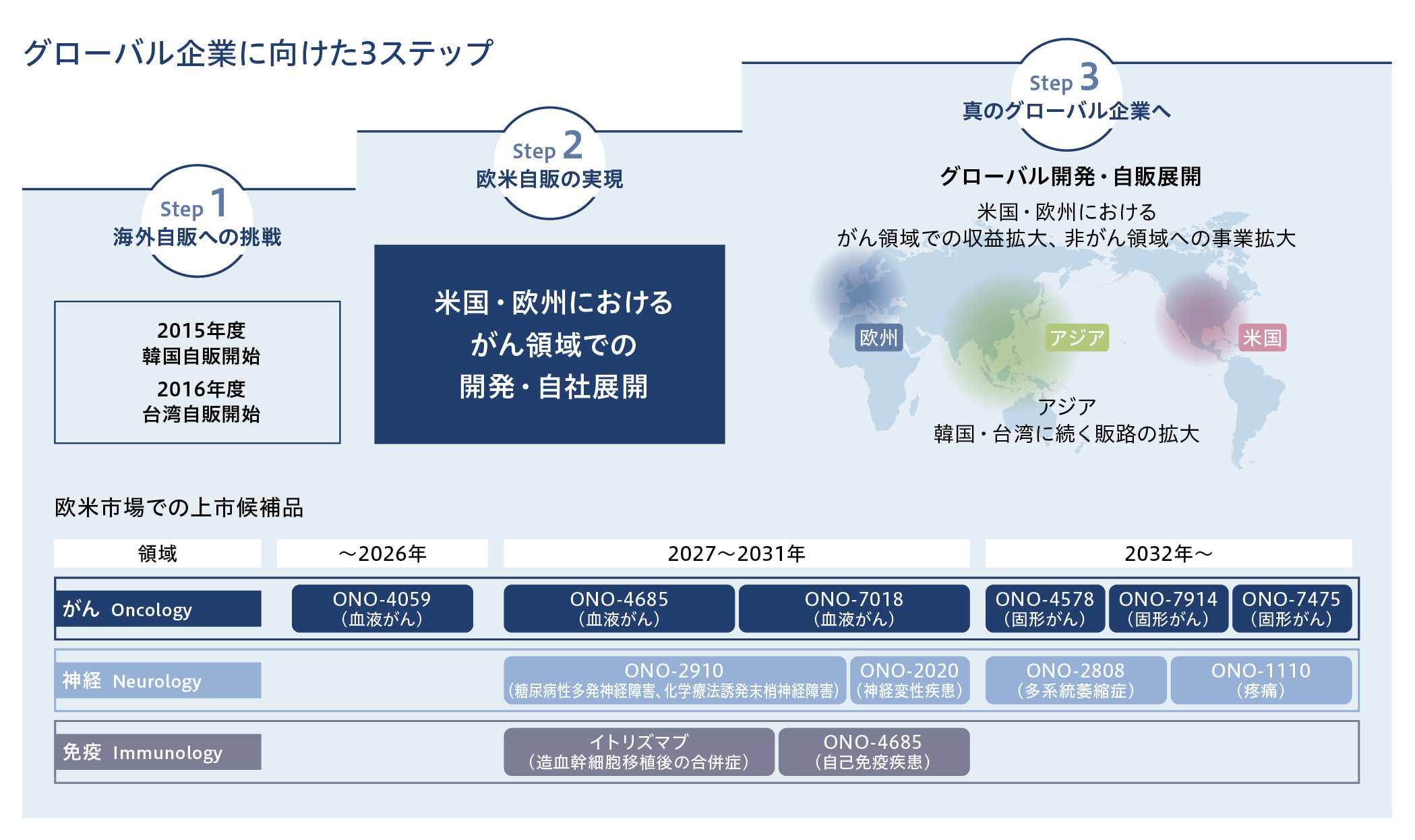
当社は、革新的な医薬品の提供を通じて、長きにわたり、より多くの人々の健康に貢献するために、グローバルスペシャリティファーマを目指しています。そのための重要戦略の一つとして、当社が創製・開発した医薬品を世界中の患者さんにお届けするために、欧米を中心とした新薬のグローバル開発・自社販売展開の体制構築を進めています。
グローバル企業に向けた3段階のステップを計画しており、第1段階の「海外自販への挑戦」では韓国と台湾において現地法人による自販を実現しています。現在は欧米での開発・自社販売展開を進める第2段階にあります。市場規模の大きい欧米市場で複数品を販売することで、さらなる成長を実現していきます。
Step 1 :海外自販への挑戦
当社は、韓国で2013年度に韓国小野薬品を、台湾で2014年度に台灣小野藥品を、それぞれ当社100%出資子会社として設立したことを機にグローバル展開を本格化させ、アジアでのプレゼンスを着実に高めています。両国では、自社による販売体制を構築し、韓国では2015年度から、台湾では2016年度から、それぞれオプジーボの販売を開始しました。
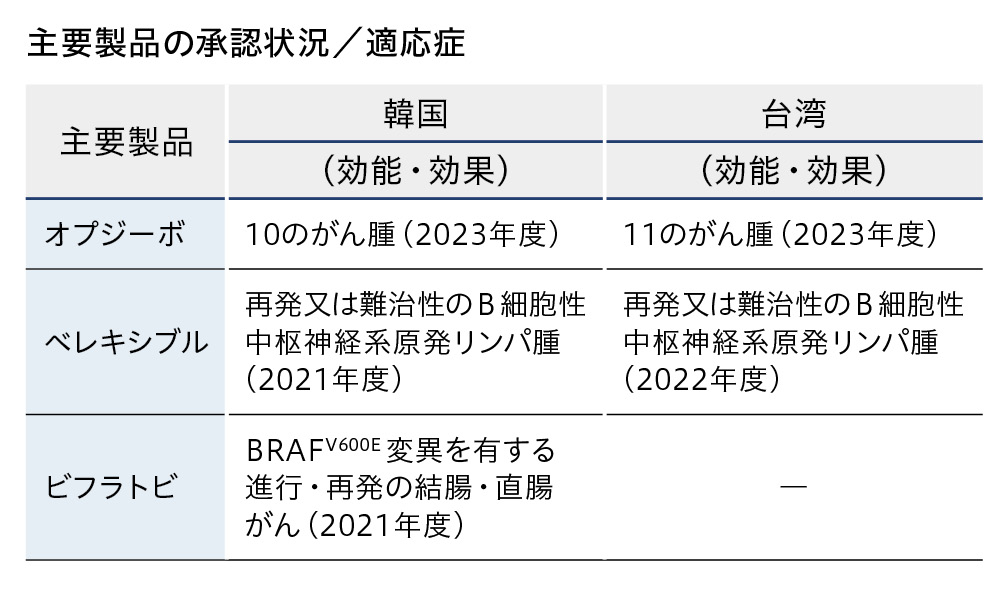
オプジーボは、韓国では10のがん腫、台湾では11のがん腫について承認を取得しています。その他の主要製品の適応症は下表の通りとなっており、両国でのがん治療の発展に寄与すべく取り組んでいます。
Step 2 :欧米自販の実現
欧米については、ONO-4059(日本製品名:ベレキシブル錠)をはじめとした複数品の販売を見据え、自社による販売体制の整備に努めています。
米国においては、2021 年、米国子会社であるONOPHARMA USA, INC.のオフィスをマサチューセッツ州に移転したことを機に、医薬品産業での経験が豊富である優秀な人財を獲得することで、競争力のある組織体制づくりを進めています。ONO-4059などの新規化合物に関する開発体制を拡充するとともに、自社販売推進に向けてコマーシャル部門、ファーマコヴィジランス部門、メディカル部門などを拡充し、2026年度に約170人規模を目安に、販売に向けた体制を強化し、ファースト・イン・クラスの製品を継続して販売していくことを目指しています。2023年、OPUSでは中枢神経系原発リンパ腫(PCNSL)に関する疾患啓発活動をASH2023(米国血液学会)より開始しました。PCNSL疾患認知度を高めることで、本疾患で悩む世界中の患者さんをサポートしていきます。
欧州では、ドイツ・イギリス・フランス・イタリア・スペインでの販売を基本とし、欧州展開を検討しています。開発部門を中心に体制の整備・強化に取り組み、後期臨床試験から承認申請までを自社で実施できる開発体制の整備に取り組んでいます。
Deciphera社を買収
中長期成長戦略である「パイプライン強化とグローバル開発の加速」および「欧米自販の実現」を見据え、2024年6月にDeciphera社を買収しました。
買収によりDeciphera社が保有するがん領域における承認/申請済みの2つの製品、開発段階にある3つの化合物を獲得し、パイプラインを拡充しました。
当社はDeciphera社の優れた研究開発能力と欧米での販売力を活かし、当社グループのパイプラインの拡充およびグローバル展開をより一層加速させるよう取り組んでいきます。
Step 3 :真のグローバル企業へ
グローバル展開の3ステップ中、第2段階に当たる「欧米自販の実現」で販売拠点を築いた地域では、さらなるアンメットニーズを満たす新薬を継続的に投入し、最後の第3段階では、特に中国・ASEANをはじめとした地域でも販売網の拡充を検討していきます。
海外拠点
- 写真は現地オフィスが入居しているテナントビルです。