Global Business

The Challenges of Pioneering the Future of Global Healthcare
Masayuki Tanigawa
Corporate Officer / Corporate Development & Strategy
The Challenges of Pioneering the Future of Global Healthcare

Masayuki Tanigawa
Corporate Officer / Corporate Development & Strategy
We are taking on the challenge of pioneering a new future for healthcare on a global scale. We have already established sales bases in Korea and Taiwan, and are moving to the second phase, which focuses on strengthening direct sales in the U.S. and European markets. In 2024, we acquired Deciphera Pharmaceuticals, Inc., significantly enhancing our new drug development and sales capabilities in oncology. Through this, by leveraging our preeminent R&D and sales capabilities, we will aim to provide innovative treatment options to patients worldwide as a true Global Specialty Pharma. Through delivering new hope and a future, we are striving for sustainable growth.
Steps for Growing as a Global Company
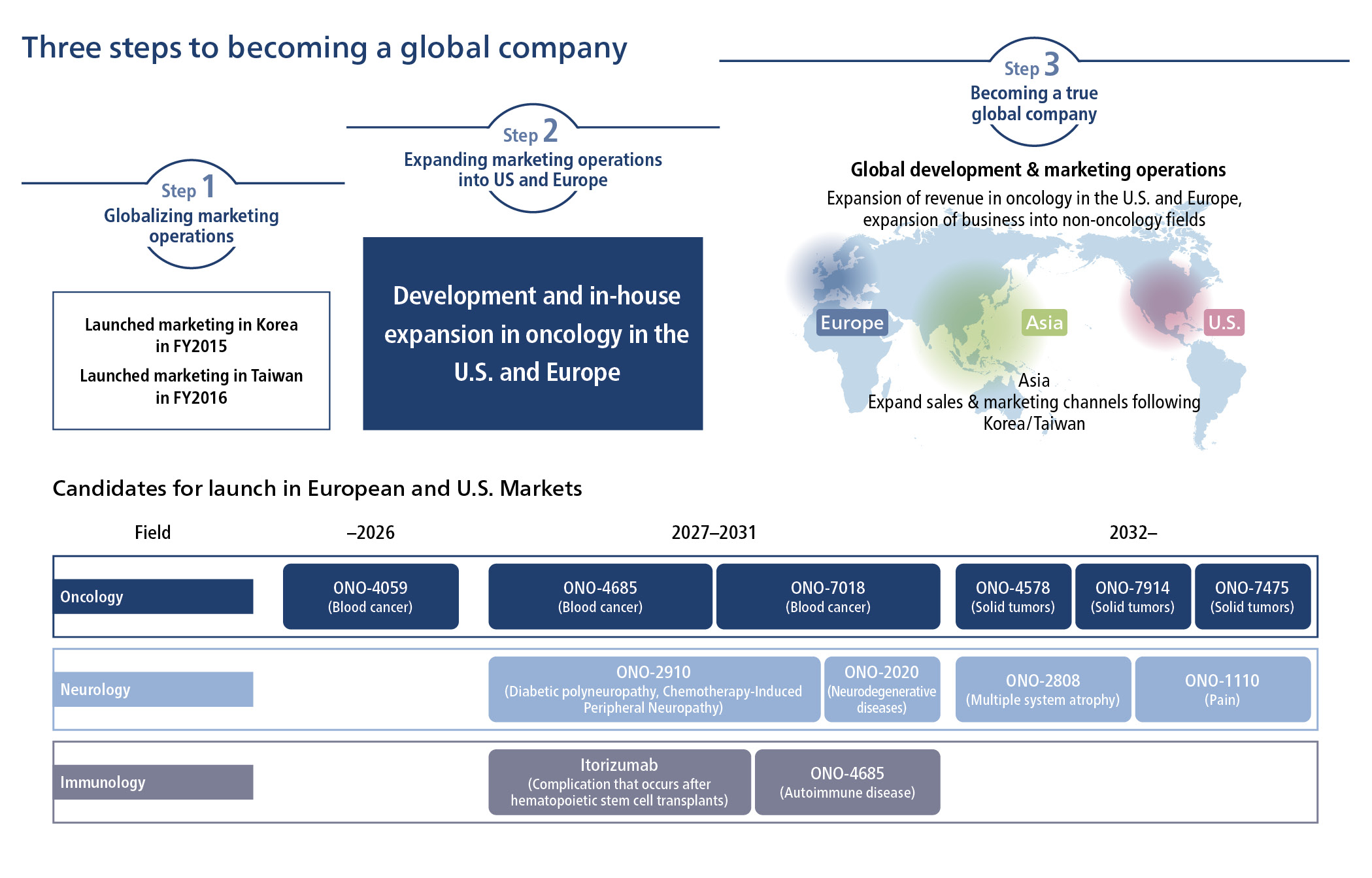
We aim to be a Global Specialty Pharma that can compete worldwide, to contribute to the health of as many people as possible over the long term, by providing innovative drugs. As one of our key strategies, in order to deliver drugs discovered and developed by our Company to patients around the world, we are working to build a system for global development and direct sales of new drugs, focusing on the U.S. and European markets. We have defined three steps that will transform us into a global company.
Step 1: “Globalizing Our Commercial Organizations” have established local subsidiaries in Korea and Taiwan, where we have already started our own sales operations. At present, we are in Step 2 working to establish our own commercial organization and direct sales operations in the U.S. and Europe. By launching a variety of products in the expansive U.S. and European markets, we will not only overcome the expiration of the OPDIVO patent but also achieve further growth.
Step 1 :Globalizing our marketing organizations
Our global expansion began in earnest with the establishment of ONO PHARMA KOREA CO., LTD. located in Korea in FY2013 followed by ONO PHARMA TAIWAN CO., LTD. in FY2014, both of which are wholly owned subsidiaries of ONO, steadily increasing our presence in Asia. The subsidiaries established their own commercial organizations, and we started direct sales operations for OPDIVO in Korea in FY2015 and in Taiwan in FY2016, respectively.
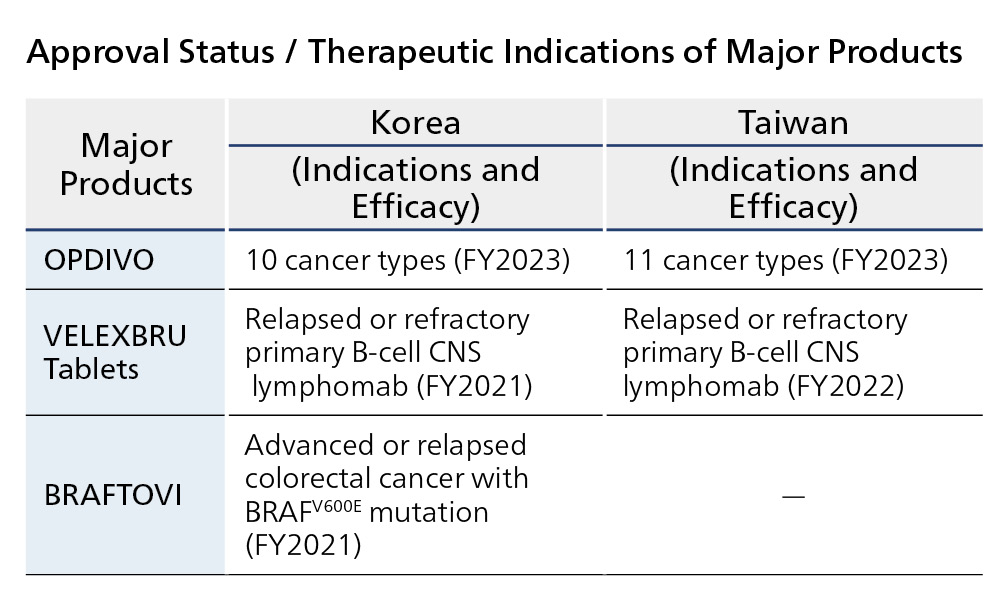
OPDIVO has been approved for 10 cancers in Korea and 11 cancers in Taiwan. The indications for other major products are as listed in the table below, and we are working to contribute to the advancement of cancer treatment in both countries.
Step 2 :Realization of direct sales in the U.S. and Europe
In the U.S. and Europe, we are working to develop our own commercial organizations for direct sales operations with an eye on launching a variety of products, such as ONO-4059 (VELEXBRU Tablets).
In the U.S., we are taking the opportunity of the office relocation of our U.S. subsidiary ONO Pharma USA, Inc. to Cambridge, Massachusetts in 2021 to acquire talented human resources with extensive experience in the pharmaceutical industry, and to create a competitive organizational structure. In addition to expanding our development structure for new drugs such as ONO-4059, we will strengthen our organization for bringing products to market by hiring human resources from Commercial, Pharmacovigilance, and Medical aiming for a team size of about 170 people by FY2026 to establish direct sales operations, and to continuously market first-in-class products. In 2023, OPUS began disease awareness activities related to primary central nervous system lymphoma (PCNSL) at ASH2023 (American Society of Hematology). By raising awareness of PCNSL, we will support patients around the world suffering from this disease.
In Europe, we are considering expanding our sales capability, focusing on sales in Germany, the United Kingdom, France, Italy, and Spain. We will continue to improve and strengthen the organization, including development, to build a development structure so we will be able to do the work from late-stage clinical trials to regulatory filings, in-house.
Acquisition of Deciphera
With a view to our medium- to long-term growth strategies of “Reinforcement of pipelines and acceleration of global development” and “Realization of direct sales in the U.S. and Europe,” we acquired Deciphera Pharmaceuticals, Inc. in June 2024. Through this acquisition, we have obtained two oncology products that have been approved or are under application, along with three drug candidates in the development stage in oncology, thereby expanding our pipeline.
We will leverage Deciphera’s excellent R&D and sales capabilities in the U.S. and Europe to further expand our pipeline, and accelerate global development within our Group.
Step 3 :Becoming a true global company
In regions where we have established sales bases with “Realization of Direct Sales organizations in the U.S. and Europe,” step 2 of the global expansion, we will continue to launch new drugs that meet further unmet needs, and in the final third step, we will consider expanding our sales capability to China, ASEAN countries, and other regions.
Overseas sites
- The photo shows the buildings where our local subsidiaries are located.