Since its establishment in 1717, ONO PHARMACEUTICAL has made progress for about 300 years--from the Edo, Meiji, Taisho, Showa, Heisei to Reiwa periods--in our commitment to focus on health improvement under the corporate philosophy of "Dedicated to the Fight against Disease and Pain."
We have made tireless efforts to unite human power and technology in discovering drugs that meet the needs of the times, which we believe has established us as a going concern with a 300-plus-year history, operating as a pharmaceutical company in Japan and the rest of the world.
In the wake of our 300th anniversary, we have created contents that introduce a history of our forebears' commitment. We appreciate your interest in our history.
“Fushimiya Ichibei” (Fushi-Ichi)
In the middle Edo period (circa 18-19th centuries) there were two powerful merchant clans in Doshomachi,
Osaka: one was the Konishi family, originated from Sakai (in what is now Osaka Prefecture) and the other was the Fushimiya family, who was allowed to accompany Hideyoshi Toyotomi from Fushimi, Kyoto upon construction of Osaka Castle.
In 1722, the Tokugawa shogunate established drug testing offices Wayakushu Aratamekaisho in five cities--Edo (now Tokyo), Sumpu (now Shizuoka), Kyoto, Osaka, and Sakai--to eliminate counterfeit drugs from entering the market of domestic medicines.
With a trade association of 124 apothecaries (later called drug broker association yakushu nakagainakama) in Doshomachi sanctioned as a monopolistic trade association kabunakama by the shogunate, all domestic medicines entering Osaka became subject to testing in Doshomachi, where the Osaka drug testing office was established by order of the shogunate for domestic drug authenticity verification. The position of the first president of this office was reportedly taken by Ichibei Fushimiya I, the head of the Fushimiya family, one powerful merchant clan in Doshomachi of mid Edo period.
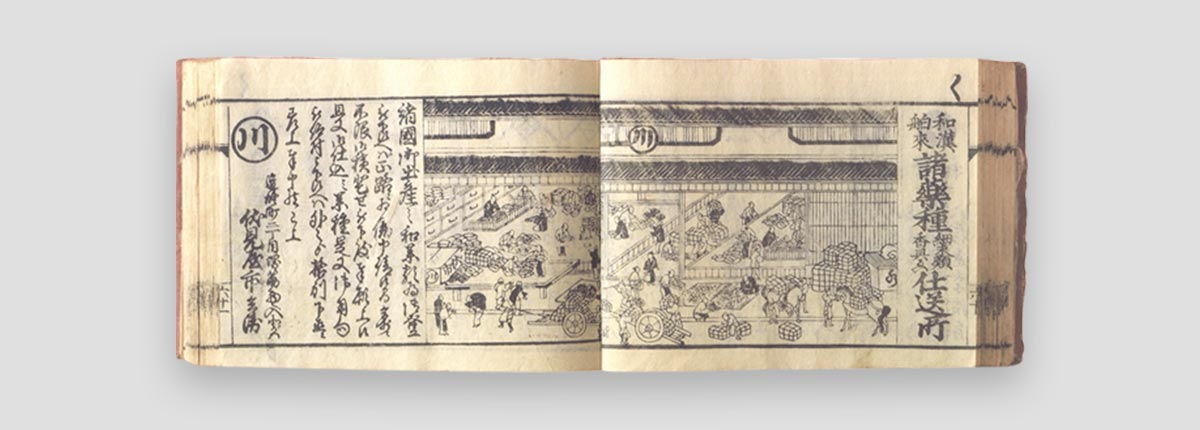
ONO PHARMACEUTICAL was originated in 1717 when Ichibei Fushimiya I opened a shop under the name “Fushimiya Ichibei” at the age of 20 with the assistance of Ichizaemon Fushimiya whom he had been apprenticed.
In those days it was difficult for a new shop to succeed on its own, and it is believed that for the first five years Ichibei’s business mainly served as a branch shop or subcontractor for his mentor Ichizaemon. In 1722, Ichibei opened his own completely independent shop in a rented building in Doshomachi, and an illustration of the shop accompanies an article on Fushimiya Ichibei in the 1849 Osaka guidebook Nippon Nisennen Sodekagami (1st edition #5).
Ichibei was first listed as a member of the kabunakama on the 1735 membership registry.
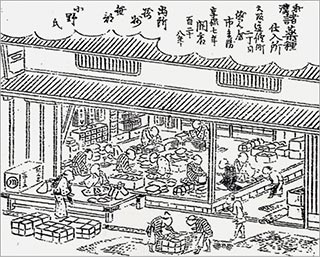
1849 Osaka guidebook Nippon Nisennen Sodekagami (1st edition #5)
Origin of Chitose Myojin (Otsuru-san)
The Coming of the Crane
In the times of Ichibei Fushimiya VI, a crane alighted on the roof of the Fushimiya shop. This episode was written in Doshomachi Manroku (by Kunijiro Mitsuhashi), which reads On October 22, 1848, the festival day of Osaka Ikasuri shrine, a crane alighted on the drying platform on the roof of the Fushimiya Ichibei shop. The glorious guest from the heaven on this auspicious day of the tutelary deity festival was immediately invited into a wire net to celebrate an auspicious sign.The scene is also depicted in the four-panel folding screen The Coming of the Crane (property of ONO PHARMACEUTICAL).
Kimpo Tanaka's Osaka Hanjo Shi describes the story of the origin of Otsuru-san.
Ichibei Fushimiya VI asked the shogunate for permission to keep the crane in the yard (tsurukakoi). The crane keeping, prohibited without shogunate permission, represented a kind of status symbol in those days. On a later day, this crane was enshrined in a corner of Ichivei Fushimi's yard as Chitose Myojin, which is now enshrined on the roof of the original headquarters.

Crane on the Warehouse Roof, a work commissioned to commemorate the coming of the crane
Fushi-Ichi to Ono Ichi
After the commencement of the Meiji period, the government actively promoted the adoption of Western medicine and pharmaceutical manufacturing technology remarkably made progress.
While other drug wholesalers were branching out into a new field of business, or pharmaceutical manufacturing, Ichibei Ono founded Dainippon Pharmaceutical Co., Ltd. (now Sumitomo Dainippon Pharma) with Chobei Takeda, Gohei Tanabe, Saburo Shionogi, and Chobei Uemura.
With joint investment by these wholesalers, this company engaged in pharmaceutical manufacturing and had a testing department, following the long-standing tradition of the drug testing office, by performing drug testing in the same status as government institutes of public health.


With the coming of the Showa Period, in 1934 Ichibei Ono VIII changed the name of the business from Fushimiya Ichibei, which had been used since it was first founded, to Ono Ichibei Shoten (Ono-Ichi), a general partnership company capitalized at 160,000 yen, and reorganized operations to modernize management.
Marketing was greatly expanded and research to develop drug manufacturing methods was begun to meet the growing demand for pharmaceutical products.
In those days, Ono Ichibei Shoten was called a "chumonya" in Doshomachi (which means a pharmacutical merchant specialized in distributing drugs to regional retailers), especially with focus on the Chugoku and Shikoku regions.
When Ono Ichibei Shoten was undertaking new drug development efforts in the early Showa period, with the spread of war, the economy became more under control by the government and the National Mobilization Law was officially promulgated, putting medicines under distribution control.
Yuzo Ono (later Ichibei Ono IX), a son of Ichibei Ono VIII, recalled the circumstances of those days in his book, titled My History of Tackling Disease and Pain. The book reads, "I rented a factory and launched pharmaceutical manufacturing business. However, new drug development is not a quick and easy job," describing the difficulties he experienced.
Fortunately, Ono Ichibei Shoten escaped war damage and after the war, promptly resumed operations, moving into a new stage as a pharmaceutical company.
In addition, ONO entered the OTC drug market on a full scale.
This was the start to tackle challenges to deliver medicines to more people.
ONO PHARMACEUTICAL
In 1947, when the scars of defeat in the war were still obvious, leaving the nation in devastation and chaos, Yuzo Ono (Ichibei Ono IX), then executive director, decided that "pharmaceutical production is the most suitable path to survive with the times," and ONO took up the challenge of moving into full-scale manufacture of pharmaceutical products, realizing a strongly held desire.
Establishing two affiliates--Nippon Yukikakou Co., Ltd. and Nippon Rikagaku Kogyo Co., Ltd., ONO made a fresh start as a pharmaceutical company with the dual functions of both sales and manufacturing.

Yuzo Ono (Ichibei Ono IX)

Street-corner advertising of EPHEDRINE
In following 1948, Nippon Yukikakou was renamed "ONO PHARMACEUTICAL Co., Ltd." In the same year, in collaboration with Professor Shunsuke Murahashi of Osaka University, ONO succeeded in the commercial manufacture of ephedrine, which was extremely difficult to synthesize in those days, and launched the asthma antitussive agent EPHEDRINE.
This dedicated passionate commitment to the challenge of creating new drugs established the spirit of drug discovery found at ONO PHARMACEUTICAL to this day.

Marketing of OTC Drugs
The years from 1950 to 1964 were a period when ONO PHARMACEUTICAL worked to expand its marketing of OTC drugs. This expansion was supported by active advertising campaigns.
Thanks to broadcast advertisements as well as print ones, Ono's OTC drugs attracted attention.

Photo courtesy of Minoru Shiraki

「RIKIHORMO」
ONO PHARMACEUTICAL sponsored the talk show, Meoto zenzai, hosted by the manzai comedy duo, Miyako Cho-cho and Nanto Yuji, and the comedy stage drama, Sucharaka shain, produced by Asahi Television Broadcasting Corporation. At the opening of the latter program, Mr. Minoru Shiraki, a then popular comedian, sang the words "...arteriosclerosis and high blood pressure both are caused by cholesterol buildup...," which popularized the term cholesterol. In addition, ONO used the then nationwide popular professional wrestler Rikidozan for the advertisement of the vitality enhancing agent RIKIHORMO (1961).

With a series of new product launches, ONO PHARMACEUTICAL expanded its network of sales branches. Upgrading with a focus on manufacturing, marketing and distribution, ONO strengthened its management base as a pharmaceutical manufacturer. These strenuous efforts bore fruit. The company listed its stock on the Osaka Stock Exchange Second Section in 1962 and the Tokyo Stock Exchange Second Section in next 1963.
to fully motivate yourself to try hard to reach the limits of your potential." (Yuzo Ono)
ONO PHARMACEUTICAL, without being satisfied with its success in OCT drug sales, was moving into a new field, that is, full-scale prescription drug development and manufacture.
The starting point was when the company encountered prostaglandins, which were called “dream substances.
After the World War II, ONO made a full-fledged entry into the OTC drug market. With the economy fluctuating and a National health insurance system introduced in 1961, however, the OTC drug market environment became increasingly severe. Under such circumstances, ONO explored the possibilities of transforming from a OTC drug manufacturer to a prescription drug manufacturer, by, e.g., establishing the Association for Gerontological Research in 1965 to conduct wide-ranging research on geriatric diseases, which had been increasing in incidence in Japan.
In 1965, ONO invited Professor Sune K. Bergström of Sweden’s Lund University to give a special lecture at the 9th Conference of the Association for Gerontological Research, which spurred ONO to meet the next challenge.
In his lecture, Professor Bergström stated that "prostaglandins (PGs) improve lipid metabolism, lower blood pressure through vasodilation, inhibit platelet aggregation, and have a relaxing effect on bronchiolar smooth muscle, exhibiting high bioactivity even in extremely small microgram amounts." The professor's lecture engaged the then president Yuzo Ono's heart and mind.
In fact, the linoleic acid that is the main ingredient of ATHERO, which ONO began marketing in 1958, is converted to PGs in the human body. Feeling a fateful encounter, Yuzo Ono decided to develop drugs based on PGs.

9th AGR Conference
Lecture by Professor Bergström, the winner of the 1982 Nobel Prize in Physiology or Medicine, who determined the chemical structure of PGs and clarified the mechanism of PG metabolization in humans
Exploring what had been unexplored to succeed in the synthesis of research-use PGs
In 1965, ONO PHARMACEUTICAL was still a small company with 20 researchers, sales of 4.3 billion yen and a profit of almost 200 million yen. With little development experience of prescription drugs. However, ONO started research on the then unidentified compounds called PGs.
ONO first undertook the development of research-use PGs. No method for the chemical synthesis of PGs had then yet been established, and the only method available was biosynthesis, requiring a considerable amount of effort to produce even small quantities. Since it was difficult to collect sheep seminal vesicles in Japan, which Professor Bergström used to determine the chemical structures of PGs, ONO decided to use bovine seminal vesicles instead. ONO researchers visited the meat market in Osaka but found that even experienced market workers could not identify seminal vesicles. Getting cooperation from veterinarians, therefore, they collected seminal vesicles from cattle's being disassembled.
 "
"
In a letter to Yuzo Ono in 1979, Professor Bergström wrote that "The moon has been shining on the truth of a bright future for "Ono-glandin"
Since then they spent two years collecting 500 kg of bovine seminal vesicles from 10,000 plus thousands of cattle and 1 ton of evening primrose seeds, from which they synthesized only 25 g of PGs. The costs of these materials reached as high as 50 million yen (2 million yen/g) in 1967 JPY. ONO PHARMACEUTICAL generously distributed the precious PGs gained in this way to researchers in Japan, deepening findings of PGs.
In 1967, the 1st PG Research Meeting was held, headed by Professor Osamu Hayaishi of Kyoto University. At this meeting, Yuzo Ono remarked: "Choosing PGs as a development target means that you are expected to encounter great difficulties ahead. At the current stage, I can only describe it as gambling. To put it exaggeratedly, I feel like Columbus sailing on the Santa Maria westward across the Atlantic Ocean in search of the New World."
However, all the first people who did what nobody else had ever done must have suffered such anxiety but if you are too anxious, you cannot come to any conclusion. Such strong determination drove ONO to move the research forward.

(Kyoto University),
who was committed to ONO's PG research
![Professor Elias J. Corey (Harvard University) [1990 Nobel winner in Chemistry], who succeeded in total chemical synthesis of PGs](/themes/onocorporate_theme/img/about/img_300th15.jpg)
[1990 Nobel winner in Chemistry],
who succeeded in total chemical synthesis of PGs
In those days, good news arrived: the success by Professor Elias J. Corey of the US’s Harvard University in total chemical synthesis of PGs. ONO immediately sent its researchers to the professor to have them learn about the chemical synthesis. Finally, in 1968, ONO became the world's first company that succeeded in the total chemical synthesis of PGs.
This chemical synthesis method made it possible to advance from the manufacture of research-use PGs to pharmaceutical grade PGs, marking a major step forward for ONO’s PG research.
Central Research Institute Moving on to Full-scale PG R&D
In June 1968 ONO PHARMACEUTICAL established the Central Research Institute (now the Minase Research Institute) to pursue full-scale drug discovery including the development of PG pharmaceutical products.

the Central Research Institute (now the Minase Research Institute)

Yuzo Ono
A stone monument engraved with the words “Dedicated to the Fight against Disease and Pain” was erected on the site of the Institute. This philosophy of ONO not only expressed the Central Research Institute’s commitment to research, but also all the employees' commitment to the development of prostaglandin drugs for the benefit of people all over the world. It has also helped convey to future generations the vitally important role of those who work in the pharmaceutical industry.
In the 1960s and 1970s Japan was rocked by numerous scandals involving drug-induced birth toxicities. ONO PHARMACEUTICAL’s business as well fell under the dark shadow of the thalidomide tragedy and chloroquine retinopathy scandal. ONO was hit with scandals involving the teratogenicity caused by the thalidomide in BONBRAIN, a sedative ONO began marketing in 1960, and the retinopathy induced by accumulation in long-term users of chloroquine, a component in the renal disease drug KIDOLA that ONO began marketing in 1961.
The efficacy and safety evaluations conducted at that time were not as rigorous as those conducted today, and as a result there was insufficient assessment of side effects and other toxicities, with sometimes inadequate cautionary information provided to medical professionals leading to the spread of such problems.
Seriously reflecting on these drug-induced toxicity scandals, ONO subsequently worked to conduct rigorous efficacy and safety evaluations of all its pharmaceutical products to ensure that such problems would never happen again.
Today as well, as a company dealing in products upon which patients’ lives depend, ONO continues working to ensure and improve the safety and reliability of all its pharmaceutical products.
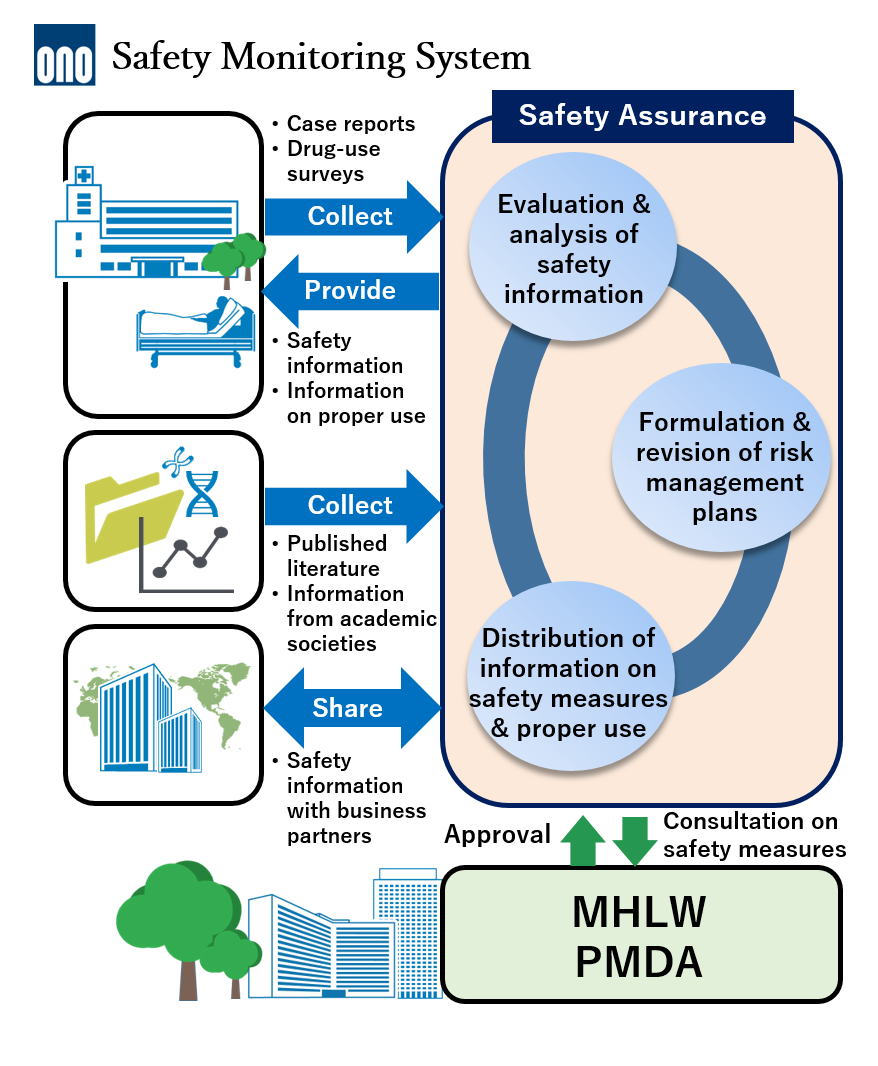
Safety Monitoring System
first PG formulation
In March 1974, nine years after ONO PHARMACEUTICAL began prostaglandin (PG) research, the labor inducing agent PROSTARMON-F Injection was launched as the world’s first PG formulation. This new drug helped to meet everyone's desire for making the painful process of childbirth and delivery much safer and smoother.
Subsequently, ONO launched the world's first products in various field; for example, the oral labor inducing agent PROSTARMON-E Tablets (1976), PROSTANDIN Injection for the treatment of intractable Buerger’s disease (1979), OPALMON Tablets for the treatment of symptoms accompanying occlusive thromboangiitis (1988).
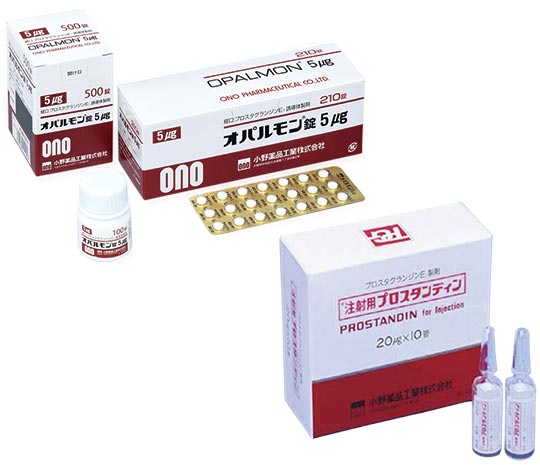
Although PROSTANDIN Injection, which is for rare disease treatment, posted questions regarding the business workability of ONO, it determined to continue development under the belief that “if there is a drug that the world needs it should be developed, and if it is a drug that is needed, then a market can be created.” Based on the policy of dedicating long-year R&D achievements to those who really need them, ONO expanded the application of PGs into various fields.
Successive Launch of the World's First Products -- Expansion of the Lineup of Original New Drugs
While launching many PG formulations on the market, ONO PHARMACEUTICAL launched FOY Injection for pancreatitis treatment in 1978 and FOIPAN Tablets, the world’s first oral protease inhibitor for chronic pancreatitis treatment, in 1985 to open a new way for pancreatitis treatment.
ONO continued bringing out the world's first original new drugs to the market: in 1988, CATACLOT for Injection, the world's first thromboxane synthase inhibitor for the treatment of ischemic symptoms after subarachnoid hemorrhage; in 1992, KINEDAK Tablets, the world’s first aldose reductase inhibitor for diabetic peripheral neuropathy treatment, and VEGA Tablets, an oral thromboxane synthase inhibitor for bronchial asthma treatment; in 1995, ONON Capsules, the world’s first leukotriene receptor antagonist for asthma treatment; and then in 2002, ELASPOL Injection, the world's first agent for acute lung injury treatment, and ONOACT Intravenous Infusion for tachyarrhythmia treatment.
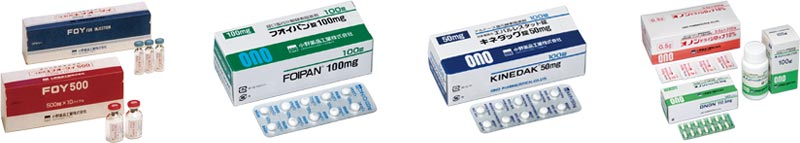
ONO continued to face challenges to be addressed.
ONO promoted open innovation through industry–academia collaboration with researchers utilizing the world’s most advanced technologies to open up the possibilities of new drug discovery.
ONO's efforts expanded to globalizing business.
Making progress with wisdom from the world
Learning from and cooperating with world-class researchers, including Professors Bergström, Samuelsson, and Vane, the 1982 Nobel winners in Physiology or Medicine, and Professor Corey, the 1990 Novel winner in Chemistry, ONO created innovative drugs.
Even before the term "open innovation" came into general use, ONO had worked on drug discovery through industry–academia collaboration with researchers utilizing the world’s most advanced technologies.
To accelerate the creation of remarkable new drugs through this open innovation, staff with drug discovery experience garnered at ONO’s research facilities are dispatched to work at ONO PHARMA UK (OPUK) and ONO PHARMA USA (OPUS). Gaining extensive local knowledge, they then visit universities, research institutes and venture companies to seek out promising themes for collaborative study. By working closely with top researchers at such organizations, ONO accelerate the drug discovery process.
ONO work to develop, through the open innovation efforts based on industry–academia cooperation, amazing new drugs in the domains with unmet medical needs and in the oncology domain.
After spending hard times, ONO PHARMACEUTICAL brought out many PG products to the market. However, following the market launch of ONOACT in 2002, development of a number of ONO’s original compounds was suspended and ONO faced major challenges. To augment the development pipeline and expand the product lineup, greater focus was placed on licensing in addition to in-house development of original compounds.
Marketing licenses were acquired from Japanese and foreign partner companies for such products as STAYBLA Tablets for overactive bladder treatment in 2007 and RECALBON Tablets for osteoporosis treatment in 2009, enabling ONO to overcome a difficult period during which it could not launch any original new products.
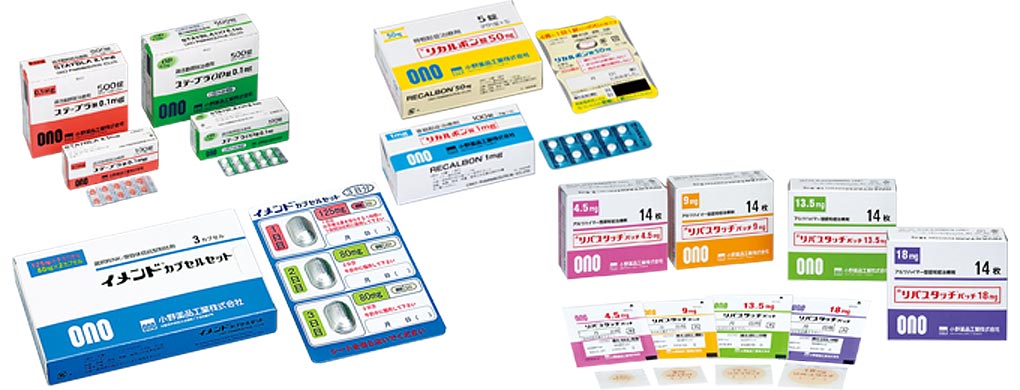
With a game-changing innovative approach of oncology, ONO are delivering hope to many patients.
There was a history of commitment: it took almost 20 years of research and development to achieve this epoch-making advance.
OPDIVO Development
When anticancer agents that attack cancer directly were predominant, OPDIVO's action mechanism was revolutionary beyond conventional cancer therapy in that the drug releases the brakes that prevent the body’s own immune system from attacking cancer cells.
In 1992, PD-1 was discovered at the Honjo laboratory of Kyoto University but its functions had long been unknown. The lab proceeded with the research persistently and in 1999, reported the crucial finding that PD-1 deficient mice develop autoimmune diseases as they age. In 2000, PD-L1 --a ligand that binds to PD-1-- was identified and in 2002, research on PD-1 deficient mice led to the discovery of PD-1's involvement in the suppression of the immune response to cancer.
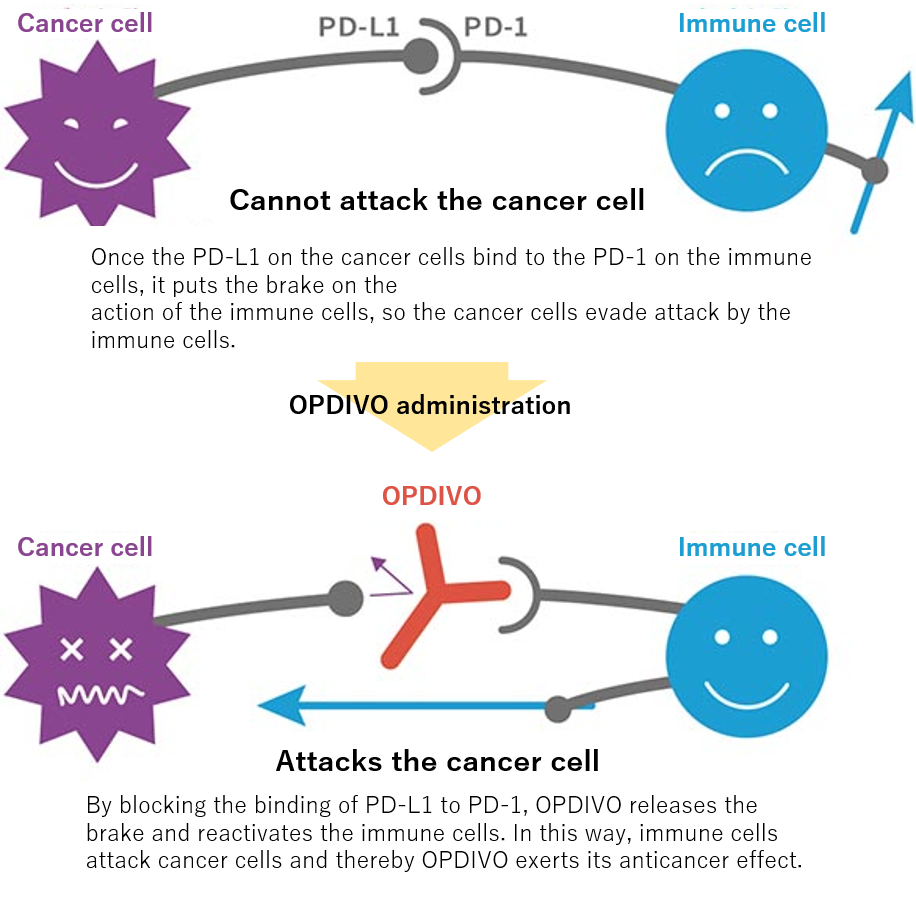
Mechanism of action of OPDIVO
For Human Beings in Fight against Cancer
After the identification of PD-1 and PD-L1, the project to develop an anti-PD-1 antibody drug shifted into high gear.
At first, however, healthcare professionals were suspicious and did not quickly accept the unprecedented concept of the development.
Medical institutions were conducting clinical trials of many anti-cancer drugs and OPDIVO was ranked 10th or lower in priority. Unfamiliar with clinical trials in oncology, the development team had days of anxiety until starting to receive reporting cases one by one where the drug worked significantly effectively. The drug rose significantly in rating among the investigators and was suddenly ranked highest in clinical trial priority.
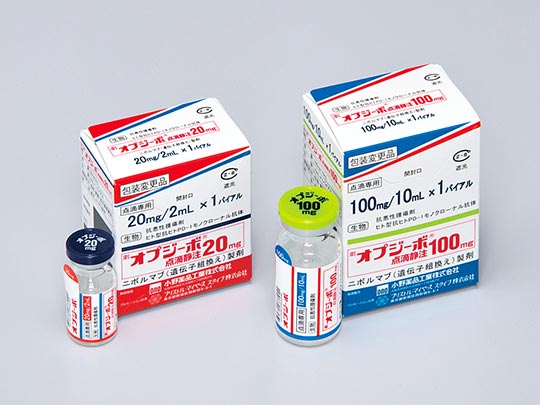
OPDIVO intravenous infusion for the treatment of malignant tumors: human anti-human PD-1
monoclonal antibody anti-cancer agent

Tsukuba Research Institute
In July 2014, OPDIVO became the first drug in the world to be authorized as an inhibitor that targets the PD-1 immune checkpoint and in September of the same year was launched on the market.
OPDIVO has an action mechanism that raises expectations for its potential to be effective, not just against specific tumors, but against a wide range of tumors.
against Disease and Pain
During 300 years since its establishment, ONO PHARMACEUTICAL has pursued new drug R&D efforts even though the success rate of new drug discovery is just one in twenty-five thousand and the drug discovery environment is becoming increasingly severe.
Experience gained from development of OPDIVO and other new drugs will lay foundation for building systems to continue creating innovation into the future. ONO PHARMACEUTICAL has made its 300-year history by continuing creating one new value after another without being satisfied with one success case.

The stone monument of the Minase Research Institute is engraved with the words of ONO's corporate philosophy,
“Dedicated to the Fight against Disease and Pain.”
We will continue to be committed to creating new drugs to deliver health to many people around the world. "Dedicated to the Fight against Disease and Pain." In accordance with this corporate philosophy, each employee will act and convert enthusiasm into tangible forms. ONO PHARMACEUTICAL now stands to step forward to a larger stage of tackling challenges.
Over 300 years:
What sort of future do you imagine?
Our world today was once the fabric of dreams.
We’ll bring to life what only Ono‘s wisdom can bring,
one step at a time and with commitment.
At Ono, we’re not like the rest. We’re surrounded by many fascinating people.
Our history of taking challenges will drive us
to passionately tackle challenges in the future.
And, that future is now,
so each of us should ask ourselves one question,
“What challenge will we take today?”
Now let's pave such a path.

