
Aiming to be a Global Specialty Pharma
Tatsuya Okamoto
Corporate Officer / Executive Director, Clinical Development
Aiming to be a Global Specialty Pharma

Tatsuya Okamoto
Corporate Officer / Executive Director, Clinical Development
– Pipeline Expansion, Strengthening Development Capabilities in the U.S. and Europe, and Maximizing Product Value –
We are moving forward with clinical development in the key areas of oncology, immunology, neurology and specialty, and focusing on pipeline expansion (strengthening), maximizing product value, and accelerating global development. To strengthen the pipeline, we are enhancing our ability to conduct trials in order to establish proof of concept (POC) at an early stage, and also incorporating various strategies to improve the accuracy of result interpretation. For products in the pipeline that have already been launched, we are maximizing product value by adding additional indications and developing new combination therapies to meet the diverse unmet needs that still exist. Additionally, we are focusing on strengthening our clinical development system in the U.S. and Europe in order to deliver to patients worldwide the drugs we have discovered and developed.
Early establishment of PoC
ONO is working to undertake speedy clinical development and improve the success rate of drug candidates in order to fast-track the delivery of our in-house and in-licensed compounds to patients suffering from diseases around the world. We are flexibly utilizing our clinical development infrastructure in Japan, the U.S. and Europe to quickly establish PoC to expediently identify the potential product value of candidates. To do this, we formulate appropriate clinical development plans, including target disease selection, propose study plans to accurately evaluate efficacy, and promote studies according to the plan. Also, while reinforcing our search for clinical markers through TR,*1 we conduct rTR*2 that links the results obtained from clinical trials to the launch of new discovery research projects by feeding those results back into research, creating an R&D virtuous cycle.
- Abbreviation of translational research. Method that applies knowledge obtained through basic research to various activities, including conducting diagnosis, treating, and determining efficacy during clinical trials.
- Abbreviation of reverse translational research. A method for feeding knowledge obtained during clinical trials back into basic research.
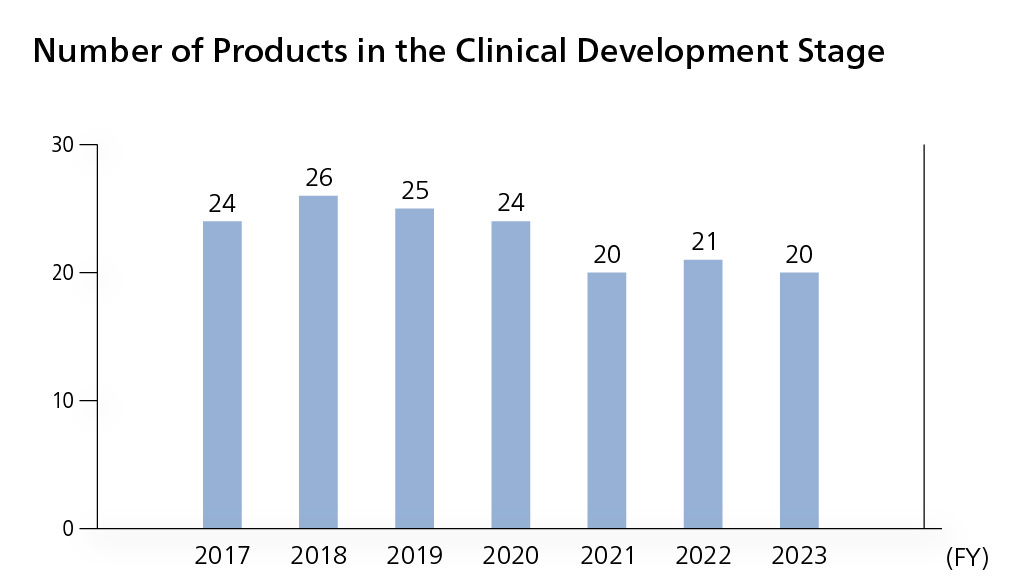
Number of products in the clinical development stage
We are moving forward with clinical development to add functions to existing products in order to increase product value. For OPDIVO, we are conducting clinical trials aimed at expanding the indications and usage for many cancers, using the drug at earlier lines of treatment, and establishing combination therapies to enhance therapeutic efficacy. We are also aggressively moving forward with global research on new compounds to reinforce our pipeline. In FY2023, there were 20 products at the clinical trial stage.
We will continue to aggressively pursue clinical development not only in Japan but also worldwide for the benefit of patients awaiting new therapeutic agents.
Acceleration of global development
Up until now, with the exception of Korea and Taiwan, where we have established our own sales systems, we have licensed out to partner companies the development and sale of drug candidates discovered in-house.
However, from now on we intend to ourselves deliver the drug candidates we have discovered and developed our-selves to patients in the U.S. and Europe, the world’s largest markets. To achieve this, we are strengthening and enhancing our clinical development system in the U.S. and Europe also, as well as conducting clinical trials targeting these countries and regions, and are building a system that can handle all processes involved in clinical development, from regulatory application to approval.
Currently, we are advancing the global development of all drug candidates discovered in-house and of those we have acquired the global commercialization rights to. In the field of oncology, we are currently conducting Phase II trials aimed at obtaining approval in the US for VELEXBRU Tablets (BTK inhibitor), which are already on the market in Japan. We are also conducting Phase II trials with ONO-4578 (EP4 antagonist) for the treatment of gastric cancer. In non-oncology, ONO-2910 (Enhancement of Schwann cell differentiation) and ONO-2808 (S1P5 receptor agonist) are both in Phase II trials, with ONO-2808 being part of a global multi-center clinical study conducted in Japan and the US. Including other products, we have 11 global development pipelines in the clinical stage.
