積極的なライセンス活動の推進
自社での創薬研究によるパイプラインの拡充に加え、国内外の製薬企業やバイオベンチャー企業などとの革新的な医薬品の創製を目的とした創薬提携活動や、開発中の新薬候補化合物の導入をめざしたライセンス活動も積極的に進めています。導入に関しては、既存製品や開発パイプラインも考慮して事業戦略性・効率性が高いと判断された化合物、あるいは医療ニーズの高い疾患に対する魅力のある化合物に注目しています。
国内外の主な提携先 (2026年2月2日現在)
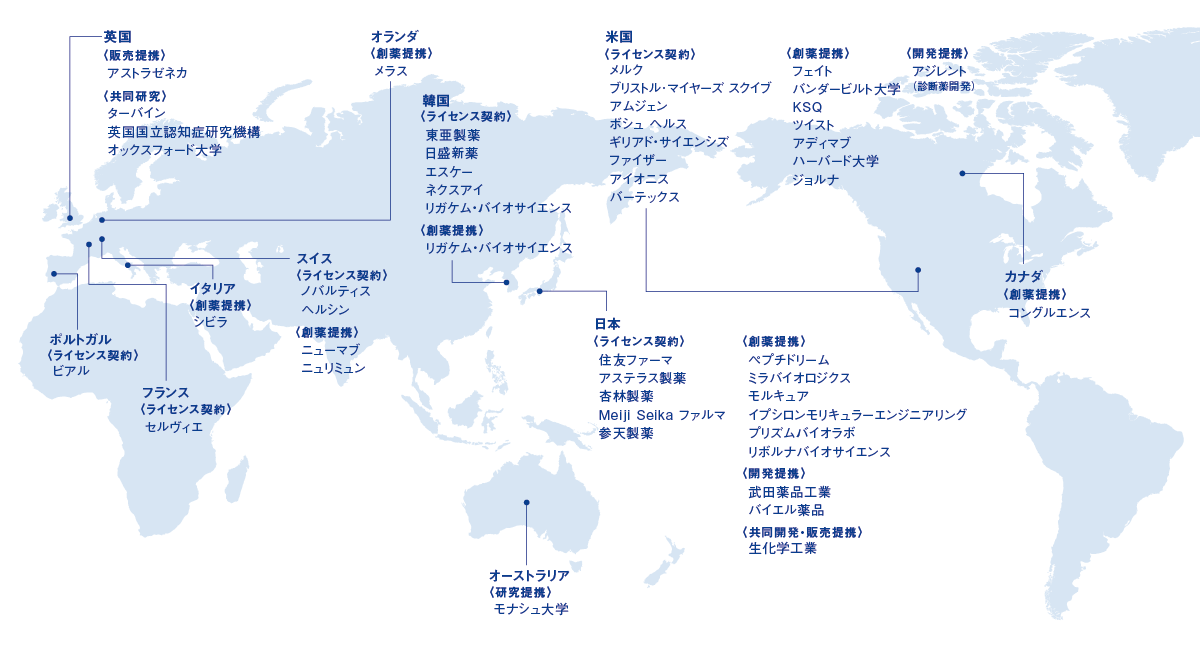
当社のこれまでの提携活動をご紹介します。国内外問わず、積極的な提携活動を推進しています。
パートナリング
当社の主な提携先をご紹介します。
なお、企業ロゴをクリックすると各社との提携内容をご覧いただけます。
導入・導出・共同販促
創薬提携・研究提携
導入・導出・共同販促
ブリストル・マイヤーズ スクイブ社 (米国)

2005年5月にメダレックス社(ブリストル・マイヤーズ スクイブ社(BMS社)が買収)とヒト型抗ヒトPD-1(programmed cell death-1)モノクローナル抗体に関する研究提携契約を締結し、その研究から生まれた「ニボルマブ(ONO-4538/BMS-936558/MDX-1106)」を創製しました。2011年9月に、ニボルマブの全世界での開発を加速させるために、BMS社の開発・販売テリトリーを日本・韓国・台湾を除く全世界に拡大する提携契約を締結しました。また、2014年7月には、日本、韓国および台湾において戦略的提携契約を締結し、複数の免疫療法薬について単剤および併用療法として共同で開発・商業化に取り組んでいます。当社は、2014年9月に日本で悪性黒色腫の治療薬として「オプジーボ®点滴静注」を発売後、さまざまながん腫での承認を取得しています。
また、ニボルマブに関する契約締結と同時に、当社は、BMS社の関節リウマチ治療剤である「オレンシア®(アバタセプト)」を日本においてBMS社の日本子会社であるブリストル・マイヤーズ スクイブ株式会社と共同で開発・販売する契約を締結し、2013年8月に「オレンシア®皮下注」の製品名で新発売しました。
ノバルティス社 (スイス)

2005年12月にノバルティス社とアルツハイマー型認知症治療剤リバスチグミン(ONO-2540/ENA713D)の日本における開発・販売に関する契約を締結しました。ノバルティス社の子会社であるノバルティス ファーマ株式会社と共同開発後、2011年7月に「軽度及び中等度のアルツハイマー型認知症における認知症症状の進行抑制」の効能又は効果で、当社は「リバスタッチ®パッチ」、ノバルティスファーマ株式会社は「イクセロン®パッチ」の製品名で新発売しました。本剤は、アルツハイマー型認知症治療剤としては初めて経皮吸収型貼付剤にしたものです。
ヘルシングループ (スイス)

2006年10月に米国のサファイア社(現在、スイスのヘルシングループの一企業であるHelsinn Healthcare SAが継承)と同社が開発中のがん悪液質治療剤アナモレリン(ONO-7643)を日本、韓国および台湾で独占的に開発・販売するライセンス契約を締結しました。アナモレリンは経口グレリン様作用薬で、がん悪液質の患者さんにおける体重および筋肉量の増加並びに食欲の増加効果を示しています。当社は、2021年1月に日本で「悪性腫瘍(非小細胞肺癌、胃癌、膵癌、大腸癌)におけるがん悪液質」の効能又は効果で製造販売承認を取得し、同年4月に「エドルミズ®錠」の製品名で新発売しました。
アムジェン社 (米国)

2010年9月にオニキス社(現アムジェン社の子会社)と、二つのプロテアソーム阻害剤「カルフィルゾミブ(ONO-7057)およびオプロゾミブ(ONO-7058)」について、全がん腫を対象に日本において独占的に開発・販売する契約を締結しました。カルフィルゾミブについては、2012年7月に米国で再発又は難治性の多発性骨髄腫の治療薬としてカイプロリス®の製品名で上市されました。日本では、2016年7月に同適応症で製造販売承認を取得し、同年8月に「カイプロリス®点滴静注用」の製品名で新発売しました。2019年11月に、既承認のデキサメタゾンとの併用療法による週 2 回投与に加え、同剤との併用療法による週1回投与の用法及び用量の追加承認を取得し、服薬利便性が向上しています。
2011年9月にカイ社(後にアムジェン社が買収)と、維持透析下の二次性副甲状腺機能亢進症を対象に開発中のカルシウム受容体作動薬「エテルカルセチド塩酸塩(ONO-5163)」について、日本で独占的に開発・販売する契約を締結しました。本剤は、副甲状腺にあるカルシウム受容体に作用して、副甲状腺ホルモンの過剰な分泌を抑制させることで、血中のカルシウム値およびリン値を低下させ、動脈硬化などの心血管系障害の発症リスクを低下させます。2016年11月に欧州で、2017年2月に米国で「慢性腎臓病に伴う血液透析患者における二次性副甲状腺機能亢進症」の適応で承認を取得しています。日本では2016年12月に「血液透析下の二次性副甲状腺機能亢進症」の適応で製造販売承認を取得し、2017年2月に「パーサビブ®静注透析用」の製品名で新発売しました。
セルヴィエ社 (フランス)

2011年9月にセルヴィエ社と、「イバブラジン塩酸塩(ONO-1162)」について、日本で独占的に開発・販売する契約を締結しました。イバブラジンは、心臓のペースメーカー機能を担うイオンチャネルの一つであるHCNチャネルを選択的に阻害し、心臓の収縮機能や血圧に影響を及ぼさずに心拍数を低下させる薬剤です。本剤は、2006年1月にセルヴィエ社により安定狭心症の治療薬として海外で発売され、2012年2月に欧州、2015年4月には米国で慢性心不全の治療薬として承認されました。日本では、当社が2019年9月に「洞調律かつ投与開始時の安静時心拍数が75回/分以上の慢性心不全(ただし、β遮断薬を含む慢性心不全の標準的な治療を受けている患者に限る。)」の効能又は効果で製造販売承認を取得し、同年11月に「コララン®錠」の製品名で新発売しました。
ビアル社 (ポルトガル)

2013年4月に、ビアル社が「パーキンソン病における症状の日内変動(wearing-off現象)」の治療薬として海外で開発中の長時間作用型COMT(カテコール-O-メチルトランスフェラーゼ)阻害剤「オピカポン(ONO-2370)」について、日本で独占的に開発・販売するライセンス契約を締結しました。本剤は、これまでの臨床試験において1日1回の服用により持続的なCOMT阻害活性が示されており、服薬利便性の向上が期待されます。欧州では、2016年6月に同剤は「レボドパ/ドパ脱炭酸酵素阻害剤(DCI)併用療法で症状が安定しないwearing-off現象が認められるパーキンソン病の患者さんにおける補助療法」として承認され、「オンジェンティス®」の製品名で販売されています。日本では、当社が2020年6月に「レボドパ含有製剤との併用によるパーキンソン病における症状の日内変動(wearing-off現象)の改善」の効能又は効果で製造販売承認を取得し、同年8月に「オンジェンティス®錠」の製品名で新発売しました。
アストラゼネカ社 (英国)

2013年12月に、アストラゼネカ社と、ナトリウム・グルコース共輸送体2(SGLT-2)阻害剤であるダパグリフロジンについて、日本におけるコ・プロモーション契約を締結しました。当社は本剤「フォシーガ®(ダパグリフロジン)錠」の日本での流通・販売を担当し、両社で共同販促を行っています。フォシーガ®は、腎尿細管でのグルコース再吸収を制御するSGLT-2に対する選択的かつ可逆的な阻害剤であり、血液中の過剰なグルコースを尿と共に体外へ排出させ、血糖を低下させる薬剤です。本剤は、インスリンを介さずに空腹時血糖および食後の高血糖を改善する2型糖尿病治療薬として承認された世界で最初のSGLT-2 阻害剤です。2014年5月に、1日1回経口投与の2型糖尿病治療薬として「フォシーガ®錠」の製品名で新発売しました。2019年3月に1型糖尿病、2020年11月に標準治療を受けている慢性心不全、2021年8月には末期腎不全又は透析施行中の患者を除く慢性腎臓病に対する効能又は効果の追加承認を取得しました。
ギリアド・サイエンシズ社 (米国)

2019年7月に米国フォーティーセブン社(2020年4月にギリアド社の完全子会社となる)と、同社が様々ながん腫を対象に開発中の抗CD47抗体であるmagrolimab(ONO-7913)について、日本、韓国、台湾およびASEAN諸国で独占的に開発および商業化するライセンス契約を締結しました。Magrolimabは、マクロファージ上のSIRPαとがん細胞上のCD47の結合を阻害する抗CD47モノクローナル抗体で、がん細胞がマクロファージからの貪食作用を回避する「don’t eat me」シグナルを無効化します。当社は日本でTP53変異陽性急性骨髄性白血病を対象とした第Ⅲ相臨床試験を骨髄異形成症候群、膵がん、結腸・直腸がんを対象とした第Ⅰ相臨床試験を実施しています。なお、ギリアド社は、海外で骨髄異形成症候群や急性骨髄性白血病などを対象に開発を進めています。
ファイザー社 (米国)

2017年5月にアレイバイオファーマ社(現ファイザーの子会社)と、BRAF阻害剤「エンコラフェニブ(ONO-7702)」とMEK阻害剤「ビニメチニブ(ONO-7703)」について、日本および韓国で独占的に開発・商業化するライセンス契約を締結しました。ビラフトビ®(エンコラフェニブ)およびメクトビ®(ビニメチニブ)は、悪性黒色腫をはじめ種々のがんに関連するMAPKシグナル伝達経路(RAS-RAF-MEK-ERK)のセリン・トレオニンキナーゼファミリーの異なるキナーゼ、BRAFおよびMEK1/MEK2をそれぞれ標的として選択的に阻害し、がん細胞の増殖を抑制します。当社は、2019年1月に日本で「BRAF遺伝子変異を有する根治切除不能な悪性黒色腫」の効能又は効果で両製剤の製造販売承認を取得し、同年2月に発売しました。2020年11月には、両製剤と抗ヒトEGFRモノクローナル抗体であるセツキシマブとの3剤併用療法およびビラフトビ®とセツキシマブとの2剤併用療法における「がん化学療法後に増悪したBRAF遺伝子変異を有する治癒切除不能な進行・再発の結腸・直腸癌」の効能又は効果の追加承認を取得しました。2023年5月、日本でビラフトビとメクトビの併用によるBRAF遺伝子変異を有する根治切除不能な甲状腺がんに対する効能又は効果の追加に係る製造販売承認事項一部変更承認申請を行いました。
生化学工業株式会社 (日本)

2017年8月に生化学工業株式会社と、同社が開発中の変形性関節症治療剤SI-613の日本における共同開発および販売提携に関する契約を締結しました。生化学工業は、糖質科学を専門分野とする研究開発型の製薬企業です。1947 年の創業以来、糖質科学の可能性に着目し、運動器疾患や眼科疾患領域において独創的で有用な医薬品・医療機器を創製し続けています。ONO-5704/SI-613(ジクロフェナクエタルヒアルロン酸ナトリウム)は、生化学工業独自の薬剤結合技術を用いてヒアルロン酸とジクロフェナク(抗炎症薬)を化学結合した薬剤です。2021年3月に生化学工業が「変形性関節症(膝関節、股関節)」の効能又は効果で製造販売承認を取得し、同年5月に当社が「ジョイクル®関節注30mg」の製品名で新発売しました。
また、2025年8月には、生化学工業株式会社が開発を進めている変形性関節症治療剤「Gel-One」の日本における共同開発および販売提携に関する契約を締結しました。
「Gel-One」は、日本において変形性関節症(膝関節)を対象とした第Ⅲ相臨床試験、変形性関節症(股関節)を対象とした第Ⅲ相臨床試験、および安全性評価を主目的とする両関節を対象とした長期投与試験を実施中です。
コーナーストーン社 (米国)

2019年6月にラファエル社(2022年5月にコーナーストーン社に名称変更)と、同社が開発中のがん代謝阻害剤であるdevimistat(ONO-7912)およびその関連化合物について、日本、韓国、台湾およびASEAN諸国での独占的な開発および商業化を目的とするライセンス契約を締結しました。コーナーストーン社は、がん代謝阻害剤の分野を牽引している企業で、治療困難ながんに有効な新規の治療薬の開発に取り組んでいます。devimistatは、細胞のミトコンドリア分画に存在し、細胞の増殖および生存に必要であるトリカルボン酸(TCA)サイクルを標的としてがん細胞を選択的に阻害し、がん細胞において様々な化学療法剤に対する感受性を大幅に高めます。この相乗効果により、低用量の化学療法剤とdevimistatを併用することで、化学療法剤の治療で一般的に認められる副作用を軽減しつつ有効性を高めることができると期待されます。
エスケー社 (韓国)

2020年10月に、SK Biopharmaceuticals Co., Ltd. (SKBP社)と同社の抗てんかん薬であるcenobamate(ONO-2017)について、日本において独占的に開発および商業化するライセンス契約を締結しました。SKBP社は、中枢神経系(CNS)疾患の治療薬の研究、開発および商業化に注力するグローバル製薬企業で、てんかんを含むCNS疾患の治療薬として8つの開発化合物で構成されるパイプラインを有しています。Cenobamateは、SKBP社とその米国子会社であるSK Life Science社によって創製・開発されました。Cenobamateが治療的効果をもたらす詳細な作用機序は不明ですが、cenobamateは電位依存性ナトリウム電流を阻害することにより、反復的な神経発火を減らすと考えられています。また、γアミノ酪酸 A型(GABAA)イオンチャネルの正の調節作用があります。Cenobamateは、2020年5月より米国で成人患者におけるてんかん部分発作の治療薬として、XCOPRI®の商品名で販売されています。また、2021年3月に欧州においても販売承認されました。日本では、てんかん強直間代発作、てんかん部分発作を対象とした第Ⅲ相臨床試験を実施しています。Cenobamateは、日本のてんかん患者さんの新たな治療選択肢になると期待されます。
コーディア社 (日本)

2020年12月に、Chordia Therapeutics株式会社(コーディア社)と、同社の粘膜関連リンパ組織リンパ腫転座1 (MALT1)阻害剤であるCTX-177およびその関連化合物について、全世界での独占的な開発、製造および商業化を目的とするライセンス契約を締結しました。コーディア社は、2017年11月にがん領域に特化した研究開発型バイオベンチャー企業として設立され、新しい作用機作を有する抗がん薬の研究と開発を行い、革新的な新薬を生み出すことを目指しています。MALT1の活性化は、リンパ球系の血液細胞のがん化に重要であることが報告されており、CTX-177 は MALT1 の活性を選択的に阻害することにより、リンパ球系の血液腫瘍に対する抗腫瘍効果が期待されます。
ライボン社 (米国)

2021年2月にライボン社と、同社が開発中のPARP7(ポリADPリボースポリメラーゼ7)阻害剤であるAtamparib(ONO-7119)について、日本、韓国、台湾およびASEAN諸国での独占的な開発および商業化を目的とするライセンス契約を締結しました。ライボン社は、細胞がストレスを受けた際に活性化する酵素を標的にしたファーストインクラスの薬剤を開発するバイオテクノロジー企業です。治療選択肢が限られた患者に有効な治療法を届けるべく、独自の創薬プラットフォームであるBEACON+を活用して新たな治療薬の開発に取り組んでいます。Atamparibは、がん細胞の生存にとって重要な役割を果たす分子であるPARP7を阻害することで、腫瘍増殖を直接抑えることに加え、がん細胞に対する免疫応答を高めるという2つのメカニズムを有することから、新たながん治療薬になることが期待されます。現在、当社は日本で固形がんを対象とした第Ⅰ相臨床試験を実施しています。
NEX-I社(韓国)

2024年3月、NEX-I社と、「NXI-101」に関するライセンス契約を締結しました。NXI-101は、NEX-I社独自の標的探索プラットフォーム(ONCOKINE®プラットフォーム)により同定されたがん免疫療法抵抗性因子ONCOKINE-1に対するファーストインクラスの抗体医薬であり、がん免疫療法抵抗性のがんを含むさまざまながん腫に対する有効性が期待されます。当社はNXI-101およびそのバックアップ抗体を全世界で独占的に開発・商業化する権利を取得します。
アイオニス社(米国)

2025年3月、米国Ionis Pharmaceuticals Inc.(Ionis社)と、同社が真性多血症の治療薬として開発中の「sapablursen」について、全世界を対象に独占的な開発および商業化するライセンス契約を締結しました。Ionis社はRNA標的医薬品のパイオニアであり、30年以上にわたり、重篤な疾患を抱える人々により良い未来をもたらす医薬品の創製に取り組んできました。Ionis社は、6つの承認された治療薬と神経、循環器、患者ニーズの高い特定領域における先進的な開発化合物パイプラインを有しています。「sapablursen」は、Ionis社によって創製・開発されたTMPRSS6を標的とした核酸医薬(アンチセンス阻害剤)です。TMPRSS6の産生を抑制し、鉄恒常性の主要な調節因子であるヘプシジンの産生を増加させることにより、真性多血症(PV)をはじめとして様々な血液疾患の治療薬となる可能性があります。Ionis社は、PVを対象とした第Ⅱ相試験の完了まで引き続き開発を担当します。当社は、その後の全世界を対象としたsapablursenの開発および商業化活動を担当します。
バーテックス社(米国)

2025年6月、米国Vertex Pharmaceuticals Incorporated(Vertex社)と、IgA腎症、原発性膜性腎症を含む複数のB細胞介在性疾患の治療薬として開発中の「Povetacicept」について、日本、韓国を対象に独占的な開発および商業化するライセンス契約を締結しました。Vertex社は、重篤な疾患に対する革新的な医薬品を創出するための科学的イノベーションに投資するグローバルバイオテクノロジー企業です。「Povetacicept」は、遺伝子組換え融合タンパク質であり、IgA腎症治療においてベストインクラスのポテンシャルを有するBAFF(B cell activating factor)およびAPRIL(a proliferation inducing ligand)に対するデュアル拮抗薬であり、IgA腎症および原発性膜性腎症患者を対象とした臨床試験において、ベストインクラスの有効性を示す可能性が示されています。また、Povetaciceptは他の自己免疫性腎疾患および自己免疫性血球減少症を含む複数の重篤なB細胞介在性疾患の治療薬としても開発中です。
メルク社 (米国)
2004年11月にメルク社より新規の経口2型糖尿病治療剤シタグリプチン(ONO-5435/MK-0431)と癌化学療法に伴う制吐剤 アプレピタント(ONO-7436/MK-0869)を導入しました。
シタグリプチンはDPP-4(ジペプチジルペプチターゼ-4)に対する選択的阻害剤で、血糖値を下げる生体内の仕組み(インクレチンシステム)を活性化することにより血糖値をコントロールする糖尿病治療薬で、日本では、当社と米メルク社の子会社であるMSD株式会社が共同開発し、2009年12月に当社は「グラクティブ錠」、MSD株式会社は「ジャヌビア錠」の製品名で発売しました。なお、欧米では米メルク社が「JANUVIA」の製品名で販売しています。
アプレピタントは、選択的ニューロキニン1受容体拮抗剤で、抗悪性腫瘍剤の投与に伴う急性及び遅発性嘔吐に有効で、日本では、当社が単独で開発し、2009年12月に「イメンドカプセル」の商品名で新発売しました。なお、欧米ではメルク社が「EMEND」の製品名で販売しています。また、2011年12月に当社は、イメンドの有効成分であるアプレピタントをプロドラッグ化した注射剤である「プロイメンド点滴静注用」を新発売し、2016年3月には生後6ヵ月以上12歳未満の小児患者さんへの使用も可能となりました。経口剤である「イメンドカプセル」の服薬が困難である患者さんがおられることや、点滴静注で投与される抗がん剤も多いことから、抗悪性腫瘍剤投与に伴う悪心・嘔吐の治療に新たな選択肢となっています。
ボシュ ヘルス社 (米国)
2013年10月にバリアント社(2018年7月にボシュ ヘルス社に名称変更)と、「デムサー®(メチロシン)」について、日本で独占的に開発・商業化する契約を締結しました。デムサーは、カテコールアミンの産生に関わるチロシン水酸化酵素を阻害することで、褐色細胞腫から過剰に分泌されるカテコールアミンを減少させ、カテコールアミン過剰分泌による高血圧、頻脈、不整脈などの症状を軽減します。日本では、当社が2019年1月に「褐色細胞腫のカテコールアミン分泌過剰状態の改善」の効能又は効果で製造販売承認を取得し、同年2月に「デムサー®カプセル」の製品名で発売しました。
創薬提携・研究提携
メラス社 (オランダ)

2014年4月、メラス社と、二重特異性抗体医薬品を共同で創製する創薬提携契約を締結し、当社は現在、この提携から取得した二重特異性抗体(ONO-4685)の臨床試験を進めています。また2018年3月、2014年4月の契約とは異なる標的で新たな創薬提携契約を締結しました。
Biclonics®と呼ばれるヒト二重特異性抗体を創製するメラス社独自の技術プラットフォームを用いて、当社が選択した創薬標的に結合する二重特異性抗体を作製し、自己免疫疾患領域における新薬候補化合物を創製することを目指しています。なお、当社は、今回の提携により創製されるBiclonics®を全世界で独占的に開発、製造および販売する権利を有しています。
ニューマブ社 (スイス)

2017年3月、ニューマブ社と、がん免疫領域において多重特異性抗体を創製する創薬提携契約およびオプション契約を締結し、2022 年3 月には、そのオプション権を行使し、新たにその多重特異性抗体を小野が全世界で独占的に開発・商業化する開発・ライセンス契約を締結しました。
また、2020年3月には新たにがん免疫領域において、2017年の契約とは異なる組み合わせの標的分子に対する新たな多重特異性抗体を創製するための創薬提携契約およびオプション契約を締結しました。
ニュリミュン社 (スイス)

2017年11月に、ニュリミュン社と、神経変性疾患領域における創薬標的に対するヒトモノクローナル抗体の創製を目的とした創薬提携契約を締結しました。ニュリミュン社独自の抗体医薬創出アプローチであるReverse Translational Medicine™(RTM™)技術を活用し、医療ニーズの高い神経変性疾患領域において当社が選択した創薬標的に対する革新的なヒトモノクローナル抗体の創製に取り組んでいます。
当社は、ニュリミュン社との創薬提携に基づいて創製される抗体医薬品を全世界で独占的に開発、商業化する権利を保有します。
フェイト社 (米国)

2018年9月、フェイト社と、がんを対象としたiPS細胞由来CAR-T細胞治療薬の創製を目的とした創薬提携契約を締結しました。
フェイト社は、がんおよび免疫疾患に対してファーストインクラスの細胞療法の開発に特化したバイオベンチャー企業です。フェイト社のiPS細胞製品プラットフォームを利用することで、繰り返し投与することができる均一な他家細胞製品を大量に生産することが可能になります。
2022年6月、当社はCAR-T細胞に加え、CAR-NK細胞療法の選択肢を新たに追加し、さらに、固形がんに対する2つ目の標的を追加して同社との提携を拡大する覚書を締結しました。当該標的に結合する抗体は当社からフェイト社に提供します。フェイト社は固形がんの2つの標的に対する細胞治療薬を創製し、当社はフェイト社が創製した細胞治療薬を全世界で開発・商業化する権利を有しており、フェイト社は欧米における共同開発・共同販売のオプション権を有しています。また2022年11月、iPS細胞由来のキメラ抗原受容体(CAR)-T細胞治療薬の創製を目的とする創薬提携契約に基づき創製したiPS細胞由来のヒト上皮細胞増殖因子受容体2(HER2)CAR-T細胞療法の製品候補品である「ONO-8250/FT825」を開発・商業化するオプション権を行使しました。
本オプション権行使により、当社およびFate社は、欧米において、「ONO-8250/FT825」を共同で開発および商業化するとともに、当社は欧米以外の全テリトリーにおいて独占的にONO-8250/FT825を開発および商業化する権利を取得します。なお、本提携から創製される製品の製造は、フェイト社が担当します。
ペプチドリーム株式会社 (日本)

2021年3月にペプチドリーム社と、同社独自の創薬開発プラットフォームシステム(PDPS:Peptide Discovery Platform System)を社内に導入することを目的とした非独占的ライセンス契約を締結しました。当社は、このPDPS(自動化プラットフォーム)を用いることで新しい創薬標的に対する高親和性ペプチドを迅速に取得し、革新的な新薬を短期間で、かつ高い成功確率で創製することを目指しています。また、2023年3月には、複数の創薬標的に対する特殊環状ペプチド医薬品の創製に関する創薬提携契約を締結しました。当社は、この新たな契約により、その医薬品候補化合物を全世界で独占的に開発・商業化する権利を取得します。
ミラバイオロジクス株式会社 (日本)

2021年8月、ミラバイオロジクス社と次世代バイオ医薬品の創製を目的とした創薬提携契約を締結しました。ミラバイオロジクス社は、2017年7月に創立された次世代多機能バイオ医薬品の創薬研究を行っているバイオベンチャー企業です。本提携において、当社とミラバイオロジクス社は、ミラバイオロジクス社独自の環状ペプチド探索法とタンパク質工学を融合させた新技術であるLassoGraft Technology®を活用し、当社が選定した複数の創薬標的を制御するバイオ医薬品の創製に共同で取り組みます。当社は、創製されたバイオ医薬品の候補化合物を全世界で独占的に開発・商業化する権利を取得しました。
モナシュ大学 (オーストラリア)

2023年1月、モナシュ大学と、自己免疫疾患および炎症性疾患の新規治療薬を創製するために、Gタンパク質共役受容体(GPCR)を標的とした抗体を創製することを目的としたオプション権付き研究提携契約を締結しました。
2024年8月、当社は前回の契約とは異なるGPCRの標的分子に対する新たな抗体を創製することを目的とした提携契約を締結しました。Monash大学のBiomedicine Discovery Instituteは、これまで標的とすることが困難であったGPCRを標的とする新たな抗体を創製します。当社は、Monash大学が創製する複数の抗体の中から、当社が選択する抗体を用いて創製した抗体医薬品を全世界で独占的に開発・商業化するためのオプション権を保有します。
モナシュ大学は、オーストラリア最大の大学です。同大学のスピンアウト企業は、気候変動のような地球規模の課題に対する持続可能な解決策を開発し、体外受精、心血管疾患、メンタルヘルスなどの科学的ブレークスルーを通じて人類を進歩させています。
KSQ社 (米国)

2023年1月、KSQ社と同社独自の創薬標的探索技術であるCRISPRomics®プラットフォーム技術を用いて特定した複数のDNA損傷応答に関わる早期創薬プログラム取得に関する契約を締結しました。
KSQ社のCRISPRomics®プラットフォーム技術は、幅広い疾患において、ゲノムスケールで、確度高く、かつ偏見を入れない創薬を可能にします。当社は、創製した医薬品候補について全世界で独占的に開発・商業化する権利を保有します。
モルキュア社 (日本)

2023年3月、モルキュア社と同社のAI創薬プラットフォーム技術を活用した複数の標的に対する革新的な抗体医薬品を創製することを目的とした創薬提携契約を締結しました。
モルキュア社のAI創薬プラットフォーム技術は、進化的分子工学、次世代シークエンシング技術、実験自動化技術、10億以上の分子情報を有する高品質な独自データに基づいて構築されています。これにより、多様で、高い親和性、特異性、機能性を持つ抗体やペプチドの設計を可能にしています。当社は、本創薬提携から創製される抗体医薬品候補を全世界で独占的に開発・商業化するオプション権を取得します。
ツイスト社(米国)

2023年8月、ツイスト社と、自己免疫疾患に対する革新的な抗体医薬品を創製することを目的とした創薬提携契約を締結しました。
ツイスト社独自の「Library of Libraries」は、天然に存在する配列に基づく、広範な合成抗体ライブラリのコレクションで、これらは抗体医薬品の標的を幅広くカバーするために、新規性の高い構造的特性と開発可能性を有しています。これを活用して、自己免疫疾患に対して当社が指定する治療標的に対する新規抗体の探索研究活動に取り組みます。
当社は、本提携から創製される抗体医薬品候補化合物を全世界で独占的に開発・製造・商業化する権利を有します。
アディマブ社(米国)

2023年9月、アディマブ社とがん領域における革新的な抗体医薬品を創製するため創薬提携契約を締結しました。
アディマブ社は当社が指定する複数の標的に対する新規治療用抗体を探索し、二重特異性抗体医薬品候補を創製します。当社は非臨床および臨床段階でアディマブ社が創製した二重特異性抗体医薬品候補の評価および開発を行います。
当社は、本提携から創製される抗体医薬品候補を全世界で独占的に開発・製造・商業化する権利を獲得するオプション権を有します。
ターバイン社(英国)

2023年10月、ターバイン社と同社のAI駆動型細胞シミュレーション技術であるSimulated CellTMによりがん領域における新規治療標的の同定および検証を実施する研究提携契約を締結しました。Simulated CellTM技術は、遺伝子とタンパク質のオミクス情報を基盤としたネットワークに、機械学習を活用して構築された仮想細胞を使ったシミュレーションプラットフォームです。このシミュレーションの情報を活用することで、製薬企業の新薬の成功確率が高まり、また有効性が期待される患者に適切な薬剤を届けることが期待できます。当社は、ターバイン社が同定した治療標的に対する医薬品候補を全世界で独占的に開発・商業化します。
英国国立認知症研究機構(UK DRI)(英国)

2023年12月、認知症等の神経変性疾患の研究を行う英国の国立認知症研究機構UK Dementia Research Institute(UK DRI)と認知症領域における新規治療標的分子の同定を目的とする共同研究契約を締結しました。当社は、UK DRIに所属する研究者に対して、当社の注目研究領域であるグリア細胞が関与する疾患や神経炎症領域などを中心に研究テーマを募集します。その中で優れた研究テーマを採択し、認知症治療薬の新規標的を同定する共同研究に資金を提供します。本共同研究期間中は、当社の神経領域におけるノウハウや知見を活用しながら共同研究を推進します。本共同研究を通じて、多様なタイプの認知症の新しい治療標的分子を同定するとともに、これらのメカニズムの解明に向けた探索研究を行います。本共同研究から同定された新規治療標的分子については、当社でその標的分子に対する医薬品候補化合物を創製し、新たな認知症治療薬の開発、商業化に向けて取り組みます。
EME社(日本)

2024年2月、株式会社Epsilon Molecular Engineering(EME社)と、革新的なVHH抗体医薬品の創製を目的とした創薬提携契約を締結しました。EME社は同社独自のヒト化VHH抗体スクリーニングプラットフォームである「The Month」を活用して、当社が指定する複数の創薬標的に対する新規ヒト化VHH抗体を取得します。
当社は、本提携から創製される抗体医薬品候補を全世界で独占的に開発・製造・商業化する権利を取得するオプション権を有します。
Harvard大学(米国)

2024年3月、ハーバード大学と、新規創薬標的の検証を目指した5年間の包括的研究提携契約を締結しました。
今回の提携では、当社のオープンイノベーション推進の一環として、ハーバード大学の技術開発部門であるOffice of Technology Development(OTD)と協働し、当社の重点研究領域であるがん、免疫、神経およびスペシャリティ領域でアンメットメディカルニーズの高い疾患を中心に、ハーバード大学に所属する研究ラボから新規創薬標的分子の検証に至る研究テーマを募集します。当社は、その中からハーバード大学と共同で研究テーマを採択し、採択された研究テーマについて、当社およびハーバード大学は両者の専門技術、創薬ノウハウや知見を活用しながら、共同研究を推進します。
Sibylla Biotech社(イタリア)

2024年3月、シビラ社と、神経疾患に対する新規医薬品候補化合物の創製を目的とした提携契約を締結しました。シビラ社は、同社独自のタンパク質分解技術プラットフォームであるPPI-FIT技術を活用して、タンパク質の折り畳みに干渉して分解を誘導する低分子化合物(FIDs:Folding Interfering Degraders)を同定します。当社は、本提携で同定された化合物をもとに医薬品候補を創製し、全世界で独占的に開発・商業化する権利を保有します。
オックスフォード大学(英国)

2024年3月、オックスフォード大学と革新的な医薬品の創出に向けた創薬シーズの検証と化合物の取得を目的とする包括的な創薬提携契約を締結しました。
本提携では、オックスフォード大学が有する創薬シーズの中から、当社の研究重点領域テーマに合致する創薬シーズを当社が選択し、オックスフォード大学においてその創薬シーズの検証試験および化合物のスクリーニングを実施します。先ず、当社の4つの研究重点領域の1つであり、オックスフォード大学においても専門性を有する神経領域での研究テーマを選択します。本提携に基づき、オックスフォード大学は検証試験と化合物スクリーニングを実施します。当社は、本提携で得られた化合物をもとに、医薬品候補化合物の創製・開発・商業化に取り組みます。
PRISM BioLab(日本)

2024年4月、プリズム社とがん領域における新規医薬品候補化合物の創製を目的とした創薬提携契約を締結しました。PRISM BioLabは、独自に開発したαヘリックス・βターン模倣技術を活用して、低分子化合物によるタンパク質間相互作用(PPI)の制御による創薬を目指している企業です。
両社は、タンパク質 / タンパク質相互作用(PPI)を標的としたPRISM社独自の低分子によるペプチド模倣技術「PepMetics®技術」を用いて、当社が開発を目指す創薬標的に対する開発候補化合物を共同で創製します。当社は創出された開発候補化合物を全世界で独占的に開発・商業化する権利を取得します。
リガケム・バイオサイエンス社(韓国)

2024年10月、LigaChem Biosciences, Inc社と固形がん領域における前臨床段階の抗体薬物複合体(ADC)であるLCB97に関するライセンス契約およびLCB独自のConjuAll™ ADCプラットフォームを用いた新規ADC創製に向けた創薬提携契約を締結しました。
LCB97は、L1細胞接着分子(L1CAM)を標的とし、複数のがんモデルマウスで強い抗腫瘍効果を示しています。また、別途、LCBのConjuAll™ ADCプラットフォームを用いた新規ADC創製に向けた創薬提携契約も締結し、複数の標的に対するADCの全世界での権利を取得します。
コングルエンス社(カナダ)

2024年12月、Congruence Therapeutics(Congruence社)と独自の創薬プラットフォームであるRevenir™を活用して、がん領域でタンパク質の複数の形態を標的とする新たな低分子化合物を創製することを目的とした創薬提携契約を締結しました。
本契約に基づきCongruence社は、独自の創薬プラットフォームであるRevenir™を活用して、新たな低分子化合物を創製します。当社は、創製された新たな低分子化合物に関して、全世界で独占的に開発・製造・商業化するオプション権を取得します。
リボルナバイオサイエンス社(日本)

2025年3月、株式会社リボルナバイオサイエンス(リボルナ社)と、中枢神経領域におけるリボ核酸(RNA)を標的とする新たな低分子化合物の創製に向けた創薬提携契約を締結しました。本契約に基づき、リボルナ社独自の創薬プラットフォームを用いて、RNAを標的とした医薬品候補となる低分子化合物の取得を目的とした創薬研究を共同で実施します。当社は、創製された低分子化合物に関して、全世界で独占的に開発・製造・商業化するオプション権を取得します。
ジョルナ社(米国)

2025年4月、Jorna Therapeutics社(Jorna社)と、AI技術を用いた医薬品創製に関する研究提携を開始しました。本契約に基づき、Jorna社は量子力学に基づく人工知能(AI)技術であるSkyEngineを基盤とした独自のタンパク質およびRNA生成AIモデルを用いてRNA編集医薬品の配列を設計します。当社はJorna社が設計した配列を基に、全世界で独占的に創薬、開発および商業化できるオプション権を有します。
バンダービルト大学(米国)
2015年11月、バンダービルト大学とアンメットメディカルニーズを満たす革新的な治療薬の創製を目指した創薬提携契約を締結しました。当社とバンダービルト大学は、未開拓のイオンチャネルあるいはトランスポーターが創薬標的となり得るかを検証するための化合物を見出し、その検証結果に基づいて、新規の中枢神経系疾患に対する治療薬候補の創製に取り組んでいます。
