
グローバルスペシャリティファーマを目指して
執行役員/開発本部長
岡本 達也
グローバルスペシャリティファーマを目指して

執行役員/開発本部長
岡本 達也
パイプライン拡充、米欧における開発力の強化、製品価値最大化
開発では重点領域に定めたがん、免疫、神経およびスペシャリティ領域の疾患を対象とした臨床開発を推進しており、パイプラインの拡充(強化)、製品価値最大化およびグローバル開発の加速に取り組んでいます。パイプラインの強化においてはPOCの早期確立のために試験の実行力を強化するとともに、結果解釈の精度を高めるためのさまざまな工夫を取り入れています。すでに上市を果たしたパイプラインについては、未だ満たされない多様なアンメットニーズに応えるために効能・効果の追加や新規併用療法の開発に取り組むことをもって、製品価値の最大化につなげています。また、当社が創製・開発した化合物を世界の患者さんに届けるために米国、欧州での臨床開発体制の強化に注力しています。
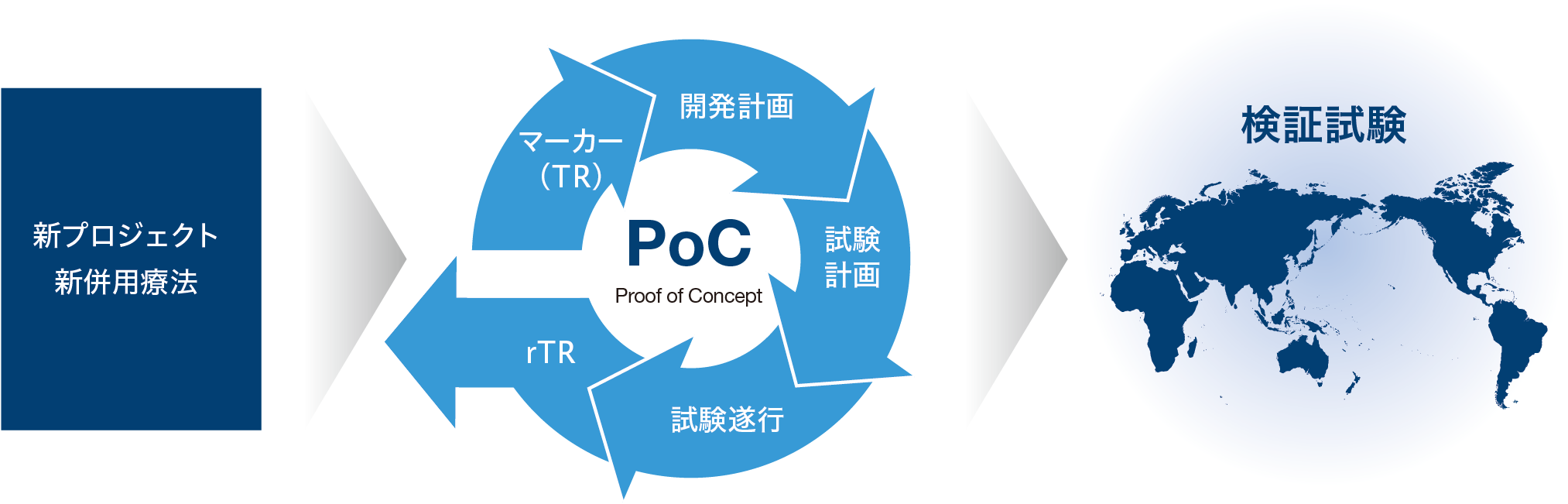
PoCの早期確立
自社の創薬研究により生み出された化合物やライセンス活動により獲得した化合物を、病気で苦しんでいる世界中の患者さんに一日も早くお届けするため、スピーディーな臨床開発と成功確率の向上に取り組んでいます。化合物の製品価値をいち早く明らかにするために、日本、米国、欧州の臨床開発基盤を柔軟に用いて、PoCの早期確立に取り組んでいます。そのために、疾患選択を含めた適切な開発計画、的確に有効性を捉えるための試験計画を立案し、試験を計画通りに推進していきます。また、TR※1による臨床マーカーの探索機能を強化し、臨床試験で得られた結果を研究にフィードバックして新たな創薬プロジェクトの立ち上げに結び付けるrTR※2にも取り組み研究開発のサイクルを回していきます。
- TR:Translational Research の略。基礎研究で得られた知見を臨床における診断、治療および効果判定などに応用する手法。
- rTR: Reverse Translational Research の略。臨床で得られた知見を基礎研究にフィードバックする手法。
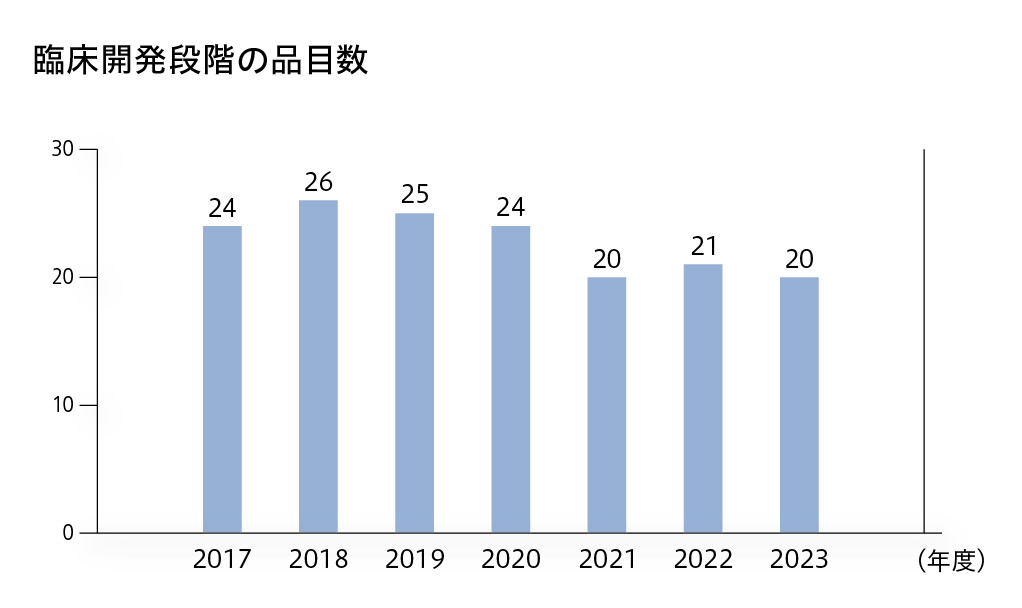
臨床開発段階の品目数
製品価値を高めるために既存製品の効能追加を目指した臨床開発も進めています。オプジーボについては、適応がん腫の拡大、より早い治療段階からの使用、治療効果を高めるための併用療法の確立を目指した臨床試験を実施しています。また、パイプラインを強化するために新規化合物のグローバル開発を積極的に進めています。2023年度に臨床段階にある品目数は20品目でした。
今後も、新たな治療薬を待ち望む患者さんのために、国内のみならず全世界で積極的に臨床開発を進めていきます。
グローバル開発の加速
これまで海外においては、自販体制を確立している韓国と台湾を除いて、自社創製の化合物の開発および販売をパートナー企業にライセンスアウトしてきました。
しかし、これからは、世界最大のマーケットを持つ米国および欧州においても、当社が創製・開発した化合物を自らの手で患者さんにお届けするようにしたいと考えています。そのために、米国および欧州においても、当該国・地域を対象とした臨床試験の実施はもちろんのこと、承認申請から承認取得に至るまでを含む臨床開発に係る工程全般を担える体制を構築し、その強化・整備を進めています。
現在は、すべての自社創製の化合物およびグローバルでの商業化の権利を獲得した導入品について、グローバルでの開発を進めています。がん領域では、日本ですでに販売しているベレキシブル錠(BTK 阻害作用)が、米国での承認取得に向けた第Ⅱ相試験の段階にあります。また、ONO-4578(EP4拮抗作用)は胃がんを対象とした第Ⅱ相試験を実施中です。非がん領域ではONO-2910(シュワン細胞分化促進作用)、ONO-2808(S1P5受容体作動作用)が、それぞれ第Ⅱ相試験の段階にあり、このうちONO-2808については日米を実施国とする国際共同試験を実施中です。その他を含め、11のグローバル開発品が臨床段階にあります。
