Initiation of Providing "Fukusapo", an Adverse Drug Reaction Management Supporting Tool for Patients Receiving Treatment with Immune Checkpoint Inhibitors
Ono Pharmaceutical Co., Ltd. (Osaka, Japan; Representative Director, President, Gyo Sagara; “ONO”) and 3H Clinical Trial Inc. (Tokyo, Japan; Representative Director, Hirotaka Takizawa; “3H”) announced that the companies developed and started providing "Fukusapo", an adverse drug reaction (ADR) management supporting tool for patients as from today, aiming at the ADR management in patients receiving the treatment with immune checkpoint inhibitors (ICIs) including ONO’s anti-PD-1 antibody/antineoplastic agent "Opdivo® (nivolumab)".
Fukusapo is a supporting tool to help early detection and treatment of ADR, especially immune-related adverse events (irAEs), through the management of physical condition of patients receiving the treatment with ICIs.
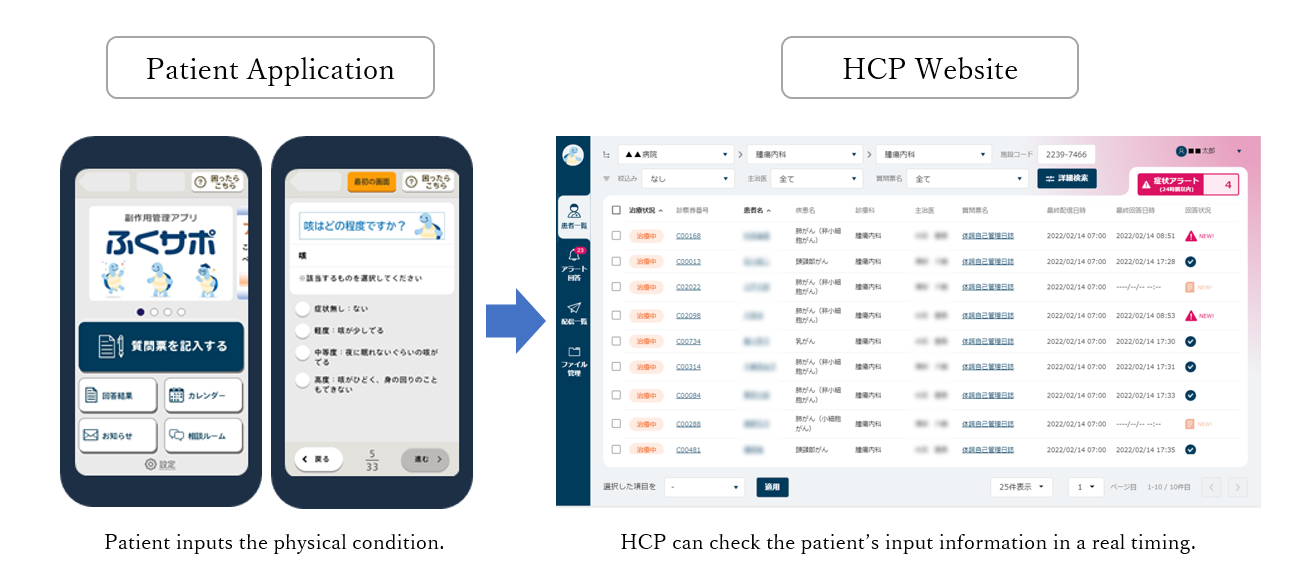
Fukusapo is based on 3H P-Guardian that is developed by 3H as an electronic patient diary. Fukusapo consists of two systems, a “Fukusapo for Patient Application” (“the App”) for patient’s smartphone that records the daily patient’s physical condition, and a “Fukusapo for Healthcare Provider (HCP) Website” (“the Web”) that allows them to check the patient input data one by one.
By having the patient input the daily physical condition into the App, an alert will be displayed on the App to encourage the patient to contact a medical institution, when ADR, especially symptoms suspicious of irAEs with their degrees are recorded. Furthermore, on the Web, patient input information can be shared with HCPs in a timely manner and smoothly. In addition, it is equipped with a monitoring function that allows the family living away from the patient to check the patient's daily physical condition.
Main functions and features of Fukusapo
< the Application >
- Questionnaire function that can record the patient's daily physical condition
- Alert display function when a symptom suspicious of ADR with its degree is selected on the questionnaire
- Information function that delivers cancer-related information weekly
- Monitoring function that allows the patient’s family to check the input status and contents of the questionnaire
< the Web >
- Timely confirmation function for questionnaire input results
- Alert display function when the patient records a symptom suspicious of ADR with its degree on the questionnaire
- CSV / PDF file format output function for questionnaire input results
- Alert notification function to send specified e-mail addresses
< Features >
- Information on skin symptoms that are difficult to verbalize can be saved and shared with HCPs on photographs.
- Questionnaire input results can be displayed as a graph.
This tool is not a medical device.
irAEs due to ICI treatment
ICI exerts an antitumor effect by activating immune cells, but irAEs may occur due to excessive immune response. irAEs associated with ICI develop an inflammatory immune response in all organs throughout the body, including the skin, digestive system, endocrine system and nervous system, making it difficult to predict when they will occur. It is known that it is difficult to early detect them due to various subjective symptoms. In addition, the management of irAEs associated with ICI treatment is one of the important issues because it may become severe if detection or treatment is delayed.
As part of efforts in solving problems in ICI treatment, ONO continues to be engaged in increasing awareness in the patient’s self-management, as well as in promoting early detection and treatment of irAEs by supporting the self-management of the patient’s physical condition and ADR management during home care with Fukusapo for patients receiving ICI treatment.
Image of Fukusapo
About Ono Pharmaceutical Co., Ltd.
Ono Pharmaceutical Co., Ltd., headquartered in Osaka, is an R&D-oriented pharmaceutical company committed to creating innovative medicines in specific areas. ONO focuses its research on the oncology, immunology, neurology and specialty research with high medical needs as priority areas for discovery and development of innovative medicines. For further information, please visit the company’s website at https://www.ono-pharma.com/ .
About 3H Clinical Trial Inc.
3H Clinical Trial Inc., the M3 Group, is a life science company that connects Human Health and Happiness. We provide healthcare IT solutions through the operation of healthcare media such as the cancer information site "Oncolo" and the clinical trial information site "Seikatsu Kojo WEB", and the development of systems such as the electronic patient diary "3H P-Guardian". We are aiming to realize digital transformation based on Patient Centricity in the pharmaceutical and medical fields. For more information, please visit https://3h-ct.co.jp/ .
